Abstract
Objective
The current study aimed to compare the effectiveness of novel radiofrequency modulation (RM) therapy with a tailored physiotherapy course for patients with chronic pelvic pain (CPP) of myofascial origin, also known as myofascial pelvic pain syndrome (MPPS).
Methods
We enrolled 46 patients with myofascial CPP to compare the effectiveness of a 10-session routine physiotherapy course versus a 6-session RM with an integrated device (HIGGS) in alleviating MPPS morbidity and pelvic floor muscle (PFM) rehabilitation. The primary outcome was reduction in pelvic pain after the final session and in the follow-up period 3 months after the final intervention session.
Results
The 6-session therapy in the RM group and the manual, biofeedback, and transcutaneous electrical nerve stimulation therapies in the physiotherapy group were similarly effective in reducing pain and improving PFM endurance after the final intervention session in each group, whereas perineometer readings and PFM strength were associated with greater improvements in the physiotherapy group.
Chronic pelvic pain (CPP) is a common manifestation of a variety of mild local or referral musculoskeletal disorders, from abdominal wall myofascial pain, muscle strains, and spasms to disorders encompassing serious systematic complications of the reproductive, urinary, gastrointestinal, and neurologic systems, among others [1]. CPP is classified as chronic recurrent or persistent non-malignant pelvic pain that lasts for at least 6 months [2]. This disorder, which is estimated to affect about 14% of the female population throughout their lives [3], is usually associated with a negative impact on the cognitive, behavioral, and sexual aspects of the lives of those afflicted [2,4,5]. Therapy in CPP is usually tailored to treat its underlying etiology [2]; however, previous studies have reported that no cause of pain was identified in 30% of patients undergoing exploratory laparoscopy [6].
Myofascial pelvic pain syndrome (MPPS) is described as an etiology of CPP in women and men, which is defined by tender points and taut bands in the pelvic floor muscles (PFMs) that include palpable nodules or myofascial trigger points that cause referred pain. As a corollary, pain can affect urinary, bowel, and sexual functions in patients [7]. Musculoskeletal dysfunction and tenderness have been reported to be higher in patients diagnosed with CPP. For instance, a previous study conducted by the authors reported a positive Carnett’s sign in 50% of participants with CPP compared with controls. This finding suggests the presence of myofascial pain in CPP and a potential association between MPPS and CPP in individuals [8]. Owing to the perceived location of pelvic pain in this subset of patients, many women assume the source of the pain to be of reproductive origin and commonly seek health care from their gynecologists, oblivious to the multifactorial origin of the pain [9]. Therefore, recognition of myofascial pain as a major component of CPP [7], regardless of concomitant medical pathologies, allows for a targeted treatment approach to alleviate pain in patients with CPP. Comparably, physical therapy and pelvic floor rehabilitation have been demonstrated to significantly reduce idiopathic CPP in men [10,11]. Interestingly, this modality has been shown to be superior to pharmacological therapy in the reduction of pelvic pain [11]. Therapeutic approaches focusing on the resolution of pain by evoking myofascial trigger points have also been shown to reduce the symptoms of CPP of urologic and abdominal origin [12–14].
While there is a growing consensus on utilizing multimodal approaches for physiotherapy in the management of CPP [7], the lack of substantial evidence precludes devising a comprehensive regimen [15]. In this study, we examined the effectiveness of an established and commonly performed 3-week physiotherapy program, which consists of manual therapy [7,9], biofeedback [16], and transcutaneous electrical nerve stimulation (TENS) [17], against an alternative approach for managing pain in patients with MPPS using an integrated device that combines previously successful shortwave (radiofrequency) diathermy [18–20] with a novel radiofrequency-induced muscle contraction modality.
To this end, we recruited 46 female patients diagnosed with CPP of myofascial origin from the gynecology clinic at Yas University Hospital, Tehran, Iran to assess the efficacy of each multimodal approach in reducing perceived pain and improving PFM function.
This study was conducted at the Yas University Hospital, Tehran, Iran, between September 2019 and April 2020. The inclusion criteria were women older than 18 years who experienced recurring or constant pain in the pelvis, perineum, anterior abdominal wall below the umbilicus, or in the lower back, which was unrelated to menstruation, intercourse, or pregnancy, and lasted for at least 6 months. Exclusion criteria were participants who did not wish to participate or remain in the study, patients with non-myofascial chronic pelvic pain, pregnant women, and participants with prosthetics or those who had metal implants such as intrauterine devices. The patients were randomized into physiotherapy or radiofrequency groups (1:1 ratio) using 4-block permuted randomization in R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) (Fig. 1).
The diagnosis of CPP was based on the standard definition and recognition by a female pelvic floor medical expert. Patients were diagnosed with MPPS with a history of 6 months of noncyclic CPP, a minimum score of 5 out of 10 on the visual analog scale for subjective pain, and pain in at least 2 out of 5 pelvic floor trigger points [21] when examined under pressure of the examiner’s index finger (pressure roughly equivalent to 2 kg/cm2). Patients without MPPS were excluded through interviews, observations, physical examinations, medical consultations with other specialists, and imaging procedures for each participant prior to inclusion. The research ethics committee at the Tehran University of Medical Sciences approved this study (Approval Number: IR.TUMS.MEDICINE.REC.1398.162). Written informed consent was obtained from all participants. This trial has been registered in the Iranian Registry of Clinical Trials (IRCT20200311046746N2).
The background information and demographics of the participants were obtained using a paper-based model questionnaire completed by each recruited patient and included age, height, weight, body mass index (BMI), occupation, education, menstruation cycle, dysmenorrhea, number of pregnancies and childbirths, types of deliveries, and questions about medical history to screen for diabetes mellitus and/or coexisting cardiovascular diseases, lower back pain, CPP, and sexual dysfunction. The content validity and reliability of this model questionnaire have already been examined and approved by Dehghan et al. [22].
To assess the intra-rater reliability of clinical examinations performed by the sole certified examiner of the study, a preliminary pilot study was conducted by the physician on seven women with CPP in a single day. Agreement was calculated using Cohen’s kappa coefficient. Cronbach’s alpha values were also calculated for the quantitative variables.
Lumbar lordosis was measured with a flexible ruler using the following equation: q=4 arctan (2 h/L). The reliability and validity of this method for measuring lumbar lordosis have been thoroughly investigated in previous studies [23].
The pelvic sagittal inclination angle was measured in the standing position on the floor using an inclinometer to determine the angle formed by a horizontal line drawn between the anterior and posterior superior iliac spines. Pelvic symmetry was assessed by inspecting the symmetry of iliac crest height and pubic tubercle symmetry. The sacroiliac joint evaluation was performed using a set of pain provocation tests, including distraction, compression, thigh thrust, sacral thrust, and Gaenslen’s nutation. The reliability and credibility of these tests in the assessment of pelvic musculoskeletal function have been proven in previous studies.
The muscular origin of the pain complaints was verified using Carnett’s sign to discriminate between parietal and visceral pain after inspection for skin damage or scarring. Myofascial-originated pain was distinguished when the pain was aggravated by the pressure exerted. Physical examination revealed no concurrent skeletal disorder.
Vaginal palpation was performed in the standard lithotomy position to evaluate PFM function. First, the resting tone of the PFM (reduced, normal, or increased) was assessed. To estimate PFM strength, the women were asked to contract their PFM with maximum intensity and maintain it for 3–5 secons. Scoring was based on a modified Oxford scale [24]. PFM endurance was graded based on how long the participant could maintain PFM contraction (squeezing the examiner’s fingers during the digital exam) between 1 and 10 seconds. The results of this method for the assessment of PFM function are strongly correlated with other quantitative methods, including perineometer measurements. Pain, tenderness, spasm, and trigger points in the levator ani, obturator internus, and piriformis muscles were assessed bilaterally using the pelvic clock position exercise maneuver. The final inspection was carried out using a biofeedback device to measure relaxation tone, PFM endurance, and strength. Perineometry measurements were root mean square results of electromyography (EMG) using a 2-channel EMG of the PFM a few minutes following the digital measurements via a biofeedback device (NeuroTrac™® MYOPlus 2 Pro; Verity Medical Lid, Braishfield, UK).
Measurements were performed at three time points throughout the study: 1) immediately after inclusion in the study (baseline), 2) after the final intervention session in each group (post-intervention), and 3) during the follow-up session 3 months after the end of the intervention (follow-up). The primary outcome was the reduction in pelvic pain based on visual analog scale scores after the final session and in the follow-up period 3 months after the final intervention session. Secondary outcomes included PFM strength and endurance assessed using digital palpation and a perineometer.
The physiotherapy course utilized in this study was a 10-session treatment plan, which was run for 3 alternate days per week. The treatment consisted of local application of 20-minute TENS to areas with pain, either internally or topically, in the lower abdomen, sacrum, and/or applied intravaginally. The areas of treatment application were chosen based on both clinical examination and guidance by the patients’ referred areas of pain.
Trigger point release therapy for the levator ani, piriformis, and obturator internus muscles includes internally applied friction massage therapy. For trigger points in the lower abdomen, a massage was applied topically to the region. In the event of dyssynergy or spasm in the PFM, a biofeedback device (YSY Medical, Gallargues-le-Montueux, France) was utilized for 15–20 minutes for training and muscle relaxation.
Upon completion of TENS, biofeedback, and manual therapy in each session, the patients were given the necessary training for PFM stretching and relaxation coupled with correction of breathing pattern disorders and introduction of diaphragmatic breathing exercises, all of which were practiced under the supervision of an expert physiotherapist at the clinic. Eventually, the patients were tasked with functional exercise training to further improve muscle functions based on the progress of the treatment and alleviation of the pain during the last two sessions.
The procedures performed in the radiofrequency modulation group in this study (the group receiving the intervention hereby referred to as radiofrequency) were applied using a HIGGS device (Danesh Bonyan Maya Slim Aria Ltd., Tehran, Iran), which, according to the manufacturer, induces tissue remodeling, neovascularization, vasodilation, and elastin fiber regeneration through shortwave diathermy as well as radiofrequency-induced muscle contraction in the pelvic area. The device starts by applying radiofrequency diathermy through its “endothermy” mode (15 W heat intensity) for 15 minutes followed by its “EndoGymWarm” mode (25 W power usage) which combines radiofrequency diathermy (18.75 W heat intensity) and stimulation of pelvic muscles on a single disposable applicator pad (called intra quadratic applicator (IQA) or IQA for short) inserted intravaginally for 30 to 45 minutes in each session. A grounding pad is attached to the skin in front of the pubic tubercle. The patients underwent six treatment sessions once per week.
As this pilot study was the first to compare physiotherapy courses with radiofrequency modulation therapy, the optimal sample size per arm was proposed to be 22 with an estimated standardized difference of 0.5% and 95% upper confidence limit in a trial designed with 80% power to detect two-sided 5% significance, according to Whitehead et al. [25].
The interconnection between qualitative variables and treatment group assignment was evaluated using Pearson’s chi-square test. The statistical significance of the correlation between quantitative variables was assessed using an independent t-test or Mann-Whitney U test. Generalized estimating equations (GEE) were used to account for the adjusted causal effect of time between baseline, post-interventional data, and data at follow-up, and to compare categorical variables (presence of trigger points in abdominal wall muscles, levator ani, piriformis, and obturator internus) and quantitative variables (manually-examined PFM endurance and strength, perineometry measurements, and visual analog scale [VAS]) between the groups (radiofrequency vs. physiotherapy) by calculating the odds ratio (OR) and linear regression coefficient, respectively. A 95% confidence interval and P-value <0.05 was considered the cut off for statistical significance. The data for qualitative and quantitative variables are presented as relative frequency percentage and mean±standard deviation, respectively.
Mean values for qualitative parameters were analyzed using the Bonferroni post hoc test in all possible pairs from post-interventional data, baseline data, and follow-up for each group. Finally, subtraction of the quantitative variable values at follow-up from those at post-intervention was performed using an independent t-test.
The main study included 46 participants (22 in the radiofrequency group and 24 in the physiotherapy group) with a mean age±standard deviation of 48.89±11.50 years in all participants and 48.13±12.83 and 49.58±10.35 for the radiofrequency and physiotherapy groups, respectively. The BMI was calculated to be 4.53±27.52 and 2.06±25.55 for the radiofrequency and physiotherapy groups, respectively. Statistical analysis for homogeneity did not reveal any statistical differences in the distribution of age (P=0.675) and BMI (P=0.065) between the groups. The background data and demographics of the participants are presented in Supplementary Table 1.
The results demonstrated a 76% reduction in the presence of trigger points in the abdominal region at follow-up compared with the baseline in both groups (OR, 0.24; P<0.001). Furthermore, the odds of the presence of trigger points at the follow-up stage for the levator ani muscle was decreased compared to baseline (OR, 0.60; P<0.001), while the presence of trigger points for obturator internus during the follow-up period was reduced by 88% compared to baseline in all participants (OR, 0.12; P<0.001). Trigger points for the piriformis muscle did not differ at the follow-up stage vs. baseline measurements (P=0.148).
The analysis demonstrated a 69% reduction in abdominal trigger points at follow-up compared to baseline using routine physiotherapy compared to radiofrequency modulation (OR, 0.31; P=0.049). However, no difference was observed in the reduction of the levator ani, piriformis, and obturator internus trigger points between the groups (P>0.05) (Table 1).
The adjusted effect of time on the outcome variables at the three different time points was analyzed by comparing the follow-up data with the baseline and post-intervention data in both groups. In this regard, the mean PFM endurance score of all study participants was greater at the post-intervention time point than at baseline (OR, 0.44; P=0.001) and at follow-up compared to baseline (OR,1.40; P=<0.001). Moreover, the mean VAS scores at post-intervention (OR, 4.29; P<0.001) and follow-up (OR, 4.16; P<0.001) were significantly lower than those at baseline.
Overall, the therapeutic effectiveness of physiotherapy and radiofrequency on pain reduction and musculoskeletal function improvement was achieved by comparing PFM strength and endurance, perineometry measures, and VAS scores between the groups using GEE. The results demonstrated greater improvements in PFM strength (OR, 2.8; P=0.009) and perineometry measurements (OR, 0.46; P=0.005) in the physiotherapy group. However, there were no significant differences in VAS scores and PFM endurance between the groups (Tables 2, 3). The estimated marginal means for PFM strength and endurance, perineometry measurements, and VAS score variables at the three time points are shown in Fig. 2.
Pairwise subtraction of the outcome variables at the study time points demonstrated significant improvements in manually-examined PFM endurance (OR, 0.93; P=0.011), strength (OR, 3.07; P<0.001), and VAS score for pain (OR, 4.29; P<0.001) at the post-intervention time point compared to baseline in the radiofrequency group. The analysis also revealed that the VAS score differed between follow-up and baseline (OR, 2.50; P=0.033). Furthermore, improvements were observed in the physiotherapy group for manually-examined PFM endurance at follow-up compared to post-intervention (OR, 1.92; P<0.001) and follow-up compared to baseline (OR, 2.00; P<0.001). Moreover, manually-examined PFM strength and VAS score were also improved at follow-up (OR, 4.33; P=0.001 and OR, 5.42; P<0.001, respectively) and post-intervention (OR, 3.17; P=<0.001 and OR, 4.54; P<0.001, respectively) compared to baseline. Improvements in perineometry measurements were exclusively observed in the physiotherapy group at follow-up versus baseline (OR, 0.48; P=0.001) and at post-intervention time point versus baseline (OR, 0.26; P=0.032) (Fig. 2).
The results of this study showed comparable overall effectiveness of HIGGS radiofrequency modulation therapy versus manual therapy, biofeedback, and TENS physical therapy modalities in reducing pelvic pain upon completion of 6 and 10 sessions of therapy, respectively. In this comparative trial, VAS scores for pain in both groups were significantly reduced following treatment; however, after cessation of treatment and assessment in the follow-up period, VAS scores were further reduced in those who received physiotherapy courses, while there was a trend towards pain resurgence in those receiving radiofrequency modulation therapy that did not reach statistical significance (Fig. 2). Similar trends were also observed in outcomes for pelvic floor rehabilitation, where, unlike physical therapy, cessation of radiofrequency treatment led to a shift of the observed effects in outcomes back towards the baseline. Nevertheless, radiofrequency modulation and routine physical therapy modalities were equally beneficial in pelvic floor rehabilitation evaluated by manual assessment of PFM strength. While the analysis revealed greater efficacy of routine physical therapy modalities in improving perineometer readings, both groups failed to show significant improvement compared to their baselines.
The putative mechanisms by which radiofrequency diathermy could lead to functional improvement in PFMs and pain reduction could be roughly divided into thermal and athermal effects [26]. Heat generated through diathermy induces vasodilation and reduces muscle spasm. Pain alleviation can be attributed to either of these effects. Athermal effects result in increased cellular activity and metabolism [26,27], which may accelerate the healing process of those suffering from pain due to a remediable lesion.
Few studies have evaluated the efficacy of short-wave (radiofrequency) diathermy in the treatment of musculoskeletal or pelvic pain. Previous studies evaluating the effects of radiofrequency diathermy on back pain have demonstrated considerable improvements in the perceived pain score (VAS) and quality of life [26]. Furthermore, radiofrequency diathermy showed comparable results to analgesics in the symptomatic management of inflammatory pelvic pain [20]. The significant reduction in the VAS scores in our study also supports these findings. It is of note that the high response rate of participants to radiofrequency modulation in this study (91%, data not shown) exceeds the previous survey studies in which the thermal effects of constant or pulsed diathermy alone resulted in improvement in a limited subset of patients for soft tissue injury, low back and trigger point pain, and chronic pain relief [28].
The efficacy of physical therapy for the treatment of CPP has been established in several studies and guidelines [29]. A previous study reported significant pain alleviation in 63% of patients who received a 12-session transvaginal trigger point release course [30]. Yaraghi et. al [31] have also shown that physiotherapy courses are superior to botulinum toxin and cognitive behavioral therapy in the management of PFM spasm in vaginismus. Another study demonstrated that a 10-session intravaginal electrical stimulation therapy results in pain reduction and reduced incidence of dyspareunia up to at least 7 months after the intervention [32].
Although this preliminary study did not assess the number of latent trigger points for each muscle and was limited to documenting the presence or absence of painful trigger points in the follow-up period and at baseline, the results demonstrated a general reduction of painful trigger points in levator ani and obturator internus muscles for all participants regardless of their group assignment at follow-up, which demonstrates the effectiveness of both treatment approaches in reducing myofascial trigger points for these muscles. The diminished effectiveness of radiofrequency modulation on the reduction of abdominal trigger points was expected for the radiofrequency group, and unlike routine physiotherapy programs, abdominal wall muscles were not the primary target of these modalities.
A major limitation of this study was the insufficient sample size to account for borderline significant differences and trends observed in the current results. Given the preliminary nature of this study and the comparable post-intervention reduction in subjective pain, future studies would benefit from sample sizes with adequate statistical power to detect smaller effects, the decline of effectiveness upon treatment cessation, therapies with identical number of sessions, and differences between the effectiveness of modalities as well as control groups to rule out potential biases with subjective measurements and placebo effects of each intervention. Assessment of the effects of these modalities as adjuncts to routine physiotherapy programs or systemic administration of nonsteroidal anti-inflammatory pharmacotherapeutic agents that do not directly target visceral pain receptors [33,34] would also be interesting topics for future research.
The management of pain in patients with CPP and MPPS has been challenging even with appropriate therapies in clinical settings. The results of this study demonstrated the comparable effectiveness of a radiofrequency-based therapy plan and conventional physiotherapy programs in reducing pelvic pain and improving PFM function.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit (CRDU) of Imam Ali Hospital, Alborz University of Medical Sciences, Karaj, Iran for their support, cooperation and assistance throughout the period of study.
Notes
Ethical approval
The research ethics committee at the Tehran University of Medical Sciences approved this study (Approval Number: IR.TUMS.MEDICINE.REC.1398.162).
References
2. Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010; 57:35–48.

3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014; 17:E141–7.
4. Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, et al. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. European Urology. 2013; 64:431–9.

5. Divandari N, Manshadi FD, Shokouhi N, Vakili M, Jaberzadeh S. Effect of one session of tDCS on the severity of pain in women with chronic pelvic pain. J Bodyw Mov Ther. 2019; 23:678–82.

6. Newham AP, Van der Spuy ZM, Nugent F. Laparoscopic findings in women with chronic pelvic pain. S Afr Med J. 1996; 86:1200–3.
7. Pastore EA, Katzman WB. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs. 2012; 41:680–91.

8. Sedighimehr N, Manshadi FD, Shokouhi N, Baghban AA. Pelvic musculoskeletal dysfunctions in women with and without chronic pelvic pain. J Bodyw Mov Ther. 2018; 22:92–6.

9. Spitznagle TM, Robinson CM. Myofascial pelvic pain. Obstet Gynecol Clin North Am. 2014; 41:409–32.

10. Masterson TA, Masterson JM, Azzinaro J, Manderson L, Swain S, Ramasamy R. Comprehensive pelvic floor physical therapy program for men with idiopathic chronic pelvic pain syndrome: a prospective study. Transl Androl Urol. 2017; 6:910–5.
11. Küçük EV, Suçeken FY, Bindayl A, Boylu U, Onol FF, Gümüş E. Effectiveness of acupuncture on chronic prostatitis-chronic pelvic pain syndrome category IIIB patients: a prospective, randomized, nonblinded, clinical trial. Urology. 2015; 85:636–40.

12. Anderson RU, Wise D, Sawyer T, Nathanson BH, Nevin Smith J. Equal improvement in men and women in the treatment of urologic chronic pelvic pain syndrome using a multi-modal protocol with an internal myofascial trigger point wand. Appl Psychophysiol Biofeedback. 2016; 41:215–24.

13. Slocumb JC. Neurological factors in chronic pelvic pain: trigger points and the abdominal pelvic pain syndrome. Am J Obstet Gynecol. 1984; 149:536–43.

14. FitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012; 187:2113–8.

15. Fuentes-Márquez P, Cabrera-Martos I, Valenza MC. Physiotherapy interventions for patients with chronic pelvic pain: a systematic review of the literature. Physiother Theory Pract. 2019; 35:1131–8.

16. Chiarioni G, Nardo A, Vantini I, Romito A, Whitehead WE. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology. 2010; 138:1321–9.

17. Harvey MP, Watier A, Dufort Rouleau É, Léonard G. Non-invasive stimulation techniques to relieve abdominal/pelvic pain: is more always better? World J Gastroenterol. 2017; 23:3758–60.

18. Balogun JA, Okonofua FE. Management of chronic pelvic inflammatory disease with shortwave diathermy. A case report. Phys Ther. 1988; 68:1541–5.
19. Lamina S, Hanif S. Shortwave diathermy in the management of chronic pelvic inflammatory disease pain. J Niger Soc Phys Sci. 2008; 16:31–6.
20. Lamina S, Hanif S, Gagarawa YS. Short wave diathermy in the symptomatic management of chronic pelvic inflammatory disease pain: a randomized controlled trial. Physiother Res Int. 2011; 16:50–6.

21. Sanses TV, Chelimsky G, McCabe NP, Zolnoun D, Janata J, Elston R, et al. The pelvis and beyond: musculoskeletal tender points in women with chronic pelvic pain. Clin J Pain. 2016; 32:659–65.
22. Dehghan MF, Ghanbari Z, Foroutan M, Kouhpayehzadeh EJ, Moshtaghi Z. Chronic pelvic pain frequency among a group of Iranian employed women. Tehran Univ Med J. 2009; 66:767–73.
23. Seidi F, Rajabi R, Ebrahimi TI, Tavanai AR, Moussavi SJ. The Iranian flexible ruler reliability and validity in lumbar lordosis measurements. World J Sport Sci. 2009; 2:95–9.
24. Laycock J. Clinical evaluation of the pelvic floor. Schussler B, Laycock J, Norton P, Stanton SL, editors. Pelvic floor re-education. 2nd ed. London: Springer-Verlag;1994. p. 42–8.
25. Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016; 25:1057–73.

26. Masiero S, Pignataro A, Piran G, Duso M, Mimche P, Ermani M, et al. Short-wave diathermy in the clinical management of musculoskeletal disorders: a pilot observational study. Int J Biometeorol. 2020; 64:981–8.

28. Shields N, Gormley J, O’Hare N. Short-wave diathermy: current clinical and safety practices. Physiother Res Int. 2002; 7:191–202.

30. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013; 58:504–10.
31. Yaraghi M, Ghazizadeh S, Mohammadi F, Ashtiani EM, Bakhtiyari M, Mareshi SM, et al. Comparing the effectiveness of functional electrical stimulation via sexual cognitive/behavioral therapy of pelvic floor muscles versus local injection of botulinum toxin on the sexual functioning of patients with primary vaginismus: a randomized clinical trial. Int Urogynecol J. 2019; 30:1821–8.

32. de Oliveira Bernardes N, Bahamondes L. Intravaginal electrical stimulation for the treatment of chronic pelvic pain. J Reprod Med. 2005; 50:267–72.
Fig. 1
Flowchart of the study. Sixty participants were screened between September 2019 and March 2020 for eligibility. Forty-six patients were included in the study. TENS, transcutaneous electrical nerve stimulation.
Fig. 2
Adjusted marginal means of outcome variables in three time periods for each group assignment. PFM, pelvic floor muscle; VAS, visual analog scale.
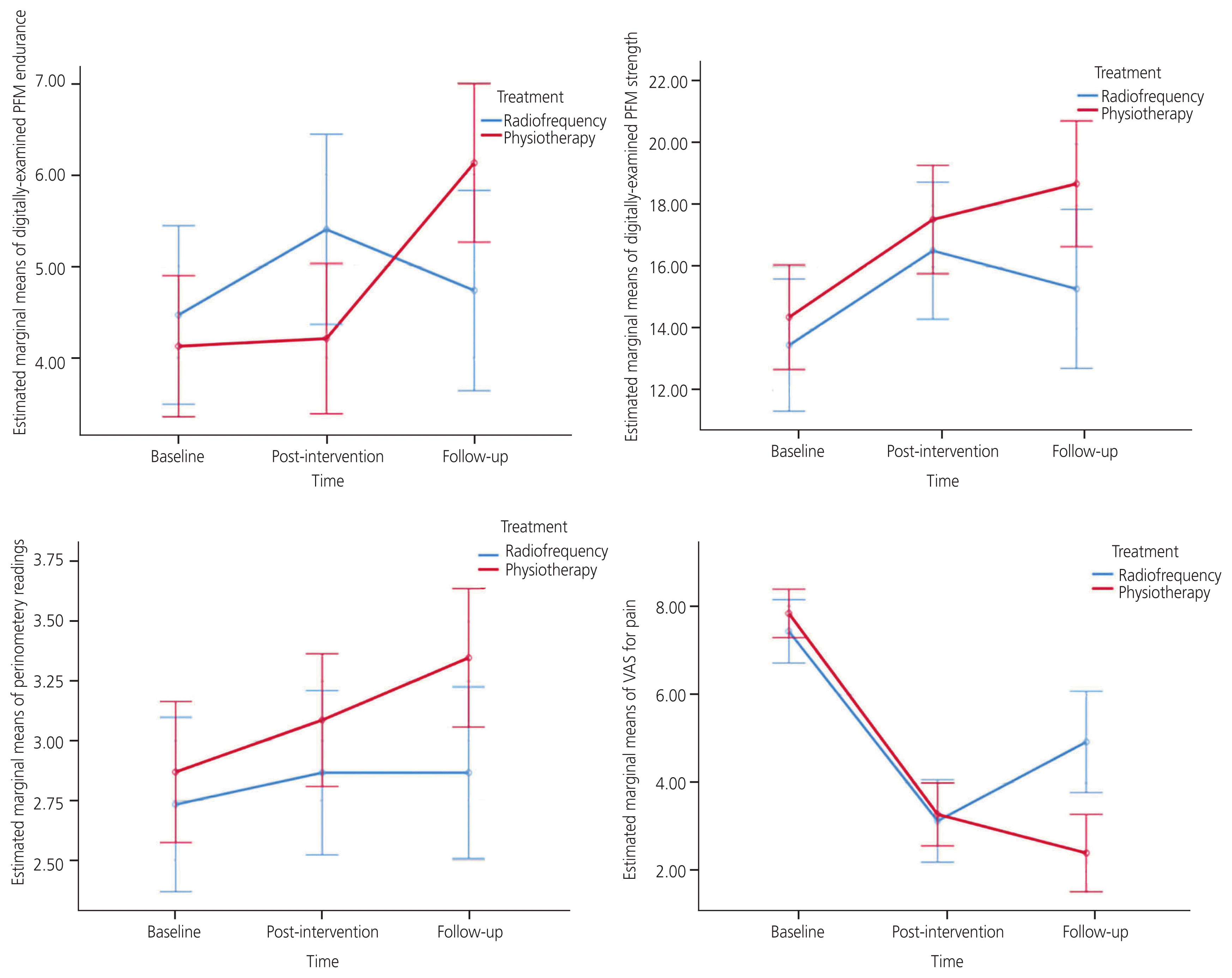
Table 1
Overall analysis of the effects of time and treatment assignment on different variables for all participants
| Outcome variable | Regression coefficient/odds ratioa) | SD | Significance | 95% CI |
|---|---|---|---|---|
| Abdominal trigger points (presence vs. absence) | ||||
| Logit | ||||
| Time (follow-up vs. baseline) | 0.24 | 0.40 | <0.001 | 0.11 to 0.52 |
| Group (physiotherapy vs. radiofrequency) | 0.31 | 0.60 | 0.049 | 0.10 to 0.99 |
| Levator ani trigger points (presence vs. absence) | ||||
| Logit | ||||
| Time (follow-up vs. baseline) | 0.60 | 0.06 | <0.001 | 0.53 to 0.68 |
| Group (physiotherapy vs. radiofrequency) | 0.96 | 0.07 | 0.550 | 0.83 to 1.10 |
| Piriformis trigger points (presence vs. absence) | ||||
| Logit | ||||
| Time (follow-up vs. baseline) | 0.15 | 1.91 | 0.148 | 0.06 to 1.18 |
| Group (physiotherapy vs. radiofrequency) | 0.98 | 0.60 | 0.973 | 0.31 to 3.15 |
| Obturator internus trigger points (presence vs. absence) | ||||
| Logit | ||||
| Time (follow-up vs. baseline) | 0.12 | 0.50 | <0.001 | 0.05 to 0.33 |
| Group (physiotherapy vs. radiofrequency) | 1.22 | 0.58 | 0.738 | 0.39 to 3.81 |
| PFM endurance | ||||
| Linear | ||||
| Time (post-intervention vs. baseline) | 0.44 | 0.13 | 0.001 | 0.18 to 0.69 |
| Time (gollow-up vs. baseline) | 1.40 | 0.31 | <0.001 | 0.80 to 2.00 |
| Group (physiotherapy vs. radio frequency) | 0.57 | 0.52 | 0.28 | −0.45 to 1.59 |
| PFM strength | ||||
| Linear | ||||
| Time (post-intervention vs. baseline) | 2.76 | 0.33 | <0.001 | 2.12 to 3.41 |
| Time (follow-up vs. baseline) | 3.15 | 0.79 | <0.001 | 1.62 to 4.67 |
| Group (physiotherapy vs. radiofrequency) | 2.80 | 1.08 | 0.009 | 0.69 to 4.91 |
| Perineometer measurements | ||||
| Linear | ||||
| Time (post-intervention vs. baseline) | 0.20 | 0.06 | 0.001 | 0.08 to 0.31 |
| Time (follow-up vs. baseline) | 0.38 | 0.12 | 0.002 | 0.14 to 0.62 |
| Group (physiotherapy vs. radiofrequency) | 0.46 | 0.16 | 0.005 | 0.14 to 0.78 |
| Pain (VAS) | ||||
| Linear | ||||
| Time (post-intervention vs. baseline) | −4.29 | 0.29 | <0.001 | −3.71 to −4.86 |
| Time (follow-up vs. baseline) | −4.16 | 0.43 | <0.001 | −3.31 to −5.00 |
| Group (physiotherapy vs. radiofrequency) | −0.60 | 0.39 | 0.124 | −1.36 to −0.16 |
Table 2
Statistical analysis of effectiveness of group-specific modalities on study endpoints compared within each group for different time points
Table 3
Pairwise comparison of effectiveness of treatment modalities of study groups at post-intervention and follow-up stages compared to baseline




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download