1. Seferović PM, Vardas P, Jankowska EA, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021; 23:906–914. PMID:
33634931.
2. Park JJ, Lee CJ, Park SJ, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021; 3:224–236. PMID:
36262554.

3. Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000; 139:72–77. PMID:
10618565.

4. Hershberger RE, Cowan J, Jordan E, Kinnamon DD. The complex and diverse genetic architecture of dilated cardiomyopathy. Circ Res. 2021; 128:1514–1532. PMID:
33983834.

5. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022; 145:e895–1032. PMID:
35363499.
6. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.

7. Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017; 47:555–643. PMID:
28955381.

8. Savarese G, Kishi T, Vardeny O, et al. Heart failure drug treatment-inertia, titration, and discontinuation: a multinational observational study (EVOLUTION HF). JACC Heart Fail. 2023; 11:1–14. PMID:
36202739.
9. Seferović PM, Polovina M, Adlbrecht C, et al. Navigating between Scylla and Charybdis: challenges and strategies for implementing guideline-directed medical therapy in heart failure with reduced ejection fraction. Eur J Heart Fail. 2021; 23:1999–2007. PMID:
34755422.

10. Wirtz HS, Sheer R, Honarpour N, et al. Real-world analysis of guideline-based therapy after hospitalization for heart failure. J Am Heart Assoc. 2020; 9:e015042. PMID:
32805181.

11. Kim E, Baek J, Kim M, Lee H, Bae JW, Kim HC. Trends in regional disparity in cardiovascular mortality in Korea, 1983-2019. Korean Circ J. 2022; 52:829–843. PMID:
36347519.

12. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022; 10:73–84. PMID:
34895860.

13. Greenberg B. Medical management of patients with heart failure and reduced ejection fraction. Korean Circ J. 2022; 52:173–197. PMID:
35257531.

14. Komajda M, Anker SD, Cowie MR, et al. Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016; 18:514–522. PMID:
27095461.

15. Khan MS, Butler J, Greene SJ. The real world of de novo heart failure: the next frontier for heart failure clinical trials? Eur J Heart Fail. 2020; 22:1786–1789. PMID:
32374051.

16. Myhre PL, Januzzi JL Jr, Butler J, Vaduganathan M. De novo heart failure: where the journey begins. Eur J Heart Fail. 2019; 21:1245–1247. PMID:
31496006.
17. Franco J, Formiga F, Corbella X, et al. De novo acute heart failure: clinical features and one-year mortality in the Spanish nationwide Registry of Acute Heart Failure. Med Clin (Barc). 2019; 152:127–134. PMID:
30712652.

18. Golla MS, Acharji S, Paul TK, Madapathi P, Garcia LA. Stent graft treatment for infra-inguinal arterial disease for either instent-restenosis and denovo lesions associated with very high rates of failure. Catheter Cardiovasc Interv. 2018; 91:1130–1135. PMID:
29214713.

19. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018; 72:351–366. PMID:
30025570.
20. von Scheidt W, Zugck C, Pauschinger M, et al. Characteristics, management modalities and outcome in chronic systolic heart failure patients treated in tertiary care centers: results from the EVIdence based TreAtment in Heart Failure (EVITA-HF) registry. Clin Res Cardiol. 2014; 103:1006–1014. PMID:
25052361.

21. Thorvaldsen T, Benson L, Dahlström U, Edner M, Lund LH. Use of evidence-based therapy and survival in heart failure in Sweden 2003-2012. Eur J Heart Fail. 2016; 18:503–511. PMID:
26869252.

22. Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF Registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016; 32:400–411. PMID:
27471353.
23. Kim IC, Youn JC, Jang SY, et al. Physician adherence and patient-reported outcomes in heart failure with reduced ejection fraction in the era of angiotensin receptor-neprilysin inhibitor therapy. Sci Rep. 2022; 12:7730. PMID:
35545653.
24. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022; 400:1938–1952. PMID:
36356631.

25. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. 2007; 50:768–777. PMID:
17707182.

26. Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND registry). Circ J. 2013; 77:944–951. PMID:
23502987.

27. Ide T, Kaku H, Matsushima S, et al. clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ J. 2021; 85:1438–1450. PMID:
33853998.

28. Zhang Y, Zhang J, Butler J, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in china: results from the China Heart Failure (China-HF) registry. J Card Fail. 2017; 23:868–875. PMID:
29029965.

29. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–1424. PMID:
32865377.
30. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. PMID:
31535829.

31. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380:539–548. PMID:
30415601.

32. Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021; 384:105–116. PMID:
33185990.
33. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382:1883–1893. PMID:
32222134.

34. Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999; 100:2312–2318. PMID:
10587334.

35. Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009; 374:1840–1848. PMID:
19922995.

36. Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996; 94:2807–2816. PMID:
8941106.

37. Hori M, Sasayama S, Kitabatake A, et al. Low-dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: the Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. Am Heart J. 2004; 147:324–330. PMID:
14760332.

38. Teng TK, Tromp J, Tay WT, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018; 6:e1008–e1018. PMID:
30103979.

39. Kim JY, Kim HJ, Jung SY, et al. Utilization of evidence-based treatment in elderly patients with chronic heart failure: using Korean Health Insurance claims database. BMC Cardiovasc Disord. 2012; 12:60. PMID:
22849621.

40. Yoo BS, Oh J, Hong BK, et al. SUrvey of Guideline Adherence for Treatment of Systolic Heart Failure in Real World (SUGAR): a multi-center, retrospective, observational study. PLoS One. 2014; 9:e86596. PMID:
24475154.

41. Kondo T, Jhund PS, McMurray JJ. Drug therapy for heart failure with reduced ejection fraction: what is the ‘right’ dose? Eur J Heart Fail. 2022; 24:421–430. PMID:
35119172.

42. Packer M, McMurray JJ. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021; 23:882–894. PMID:
33704874.

43. Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure-optimizing therapy with the need for speed. JAMA Cardiol. 2021; 6:743–744. PMID:
33787823.

44. Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–1446. PMID:
32970396.

45. Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced Trial. Circulation. 2021; 143:337–349. PMID:
33175585.

46. Chen HB, Yang YL, Yu TH, Li YH. SGLT2 inhibitors for the composite of cardiorenal outcome in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Pharmacol. 2022; 936:175354. PMID:
36306924.

47. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID:
30415602.

48. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID:
26378978.

49. Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022; 400:757–767. PMID:
36041474.

50. Chia YM, Teng TK, Tan ES, et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017; 10:e003651. PMID:
29150533.

51. Henriksen K, Battles JB, Marks ES, Lewin DI. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US);2005.
52. Erhardt L, Komajda M, Hobbs FD, Soler-Soler J. Cardiologists’ awareness and perceptions of guidelines for chronic heart failure. The ADDress your Heart survey. Eur J Heart Fail. 2008; 10:1020–1025. PMID:
18793869.

53. Verhestraeten C, Heggermont WA, Maris M. Clinical inertia in the treatment of heart failure: a major issue to tackle. Heart Fail Rev. 2021; 26:1359–1370. PMID:
32474794.

54. Yingchoncharoen T, Wu TC, Choi DJ, Ong TK, Liew HB, Cho MC. Economic burden of heart failure in Asian countries with different healthcare systems. Korean Circ J. 2021; 51:681–693. PMID:
34227265.

55. Keshvani N, Mehta A, Alger HM, et al. Heart failure quality of care and in-hospital outcomes during the COVID-19 pandemic: findings from the Get With The Guidelines-Heart Failure registry. Eur J Heart Fail. 2022; 24:1117–1128. PMID:
35289038.

56. Wonju Severance Christian Hospital. Optimize Heart Failure Care During TRANSitional Period in Patients With Acute Heart Failure (TRANS-HF) [Internet]. Bethesda (MD): U.S. National Library of Medicine;2022. cited 2023 Jan 26. Available from:
https://clinicaltrials.gov/ct2/show/NCT04900584.
57. Youn YJ, Yoo BS, Lee JW, et al. Treatment performance measures affect clinical outcomes in patients with acute systolic heart failure: report from the Korean Heart Failure Registry. Circ J. 2012; 76:1151–1158. PMID:
22343195.

58. Measuring and improving quality of care: a report from the American Heart Association/American College of Cardiology first scientific forum on assessment of healthcare quality in cardiovascular disease and stroke. Circulation. 2000; 101:1483–1493. PMID:
10736296.
59. Fonarow GC, Abraham WT, Albert NM, et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. J Card Fail. 2007; 13:722–731. PMID:
17996820.

60. Hernandez AF, Hammill BG, Peterson ED, et al. Relationships between emerging measures of heart failure processes of care and clinical outcomes. Am Heart J. 2010; 159:406–413. PMID:
20211302.

61. Bonow RO, Ganiats TG, Beam CT, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2012; 125:2382–2401. PMID:
22528524.
62. American College of Cardiology/American Heart Association Task Force on Performance Measures. Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circulation. 2008; 118:2662–2666. PMID:
19005092.

63. Heidenreich PA, Fonarow GC, Breathett K, et al. 2020 ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020; 76:2527–2564. PMID:
33153861.

64. Suzuki S, Yoshihisa A, Sato Y, et al. Clinical significance of Get With the Guidelines-heart failure risk score in patients with chronic heart failure after hospitalization. J Am Heart Assoc. 2018; 7:e008316. PMID:
30371158.

65. Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010; 3:25–32. PMID:
20123668.

66. Aktaa S, Polovina M, Rosano G, et al. European Society of Cardiology quality indicators for the care and outcomes of adults with heart failure. Developed by the Working Group for Heart Failure Quality Indicators in collaboration with the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2022; 24:132–142. PMID:
35083826.

67. Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010; 122:585–596. PMID:
20660805.

68. Fonarow GC, Yancy CW, Heywood JT. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE registry. Arch Intern Med. 2005; 165:1469–1477. PMID:
16009861.

69. Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004; 148:43–51. PMID:
15215791.

70. Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol. 2022; 79:2203–2213. PMID:
35385798.

71. National Institute for Health and Care Excellence. Chronic heart failure in adults (QS9) [Internet]. London: National Institute for Health and Care Excellence;2022. cited 2023 Jan 26. Available from:
https://www.nice.org.uk/guidance/qs9.
72. Marti CN, Fonarow GC, Gheorghiade M, Butler J. Timing and duration of interventions in clinical trials for patients with hospitalized heart failure. Circ Heart Fail. 2013; 6:1095–1101. PMID:
24046476.

73. Georgiades M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005; 96:11G–7G.

74. Lee DS, Straus SE, Farkouh ME, et al. Trial of an intervention to improve acute heart failure outcomes. N Engl J Med. 2023; 388:22–32. PMID:
36342109.

75. National Institute for Health and Care Excellence. Acute heart failure (QS103) [Internet]. London: National Institute for Health and Care Excellence;2015. cited 2023 Jan 26. Available from:
https://www.nice.org.uk/guidance/qs103.
76. Son YJ, Choi J, Lee HJ. Effectiveness of nurse-led heart failure self-care education on health outcomes of heart failure patients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020; 17:6559. PMID:
32916907.

77. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018; 101:363–374. PMID:
29102442.

78. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002; 39:83–89. PMID:
11755291.

79. Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005; 111:179–185. PMID:
15642765.

80. Jonkman NH, Westland H, Groenwold RH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail. 2016; 22:861–871. PMID:
27374838.

81. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014; 1:4–25. PMID:
28834669.

82. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004; 44:810–819. PMID:
15312864.

83. Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005; 91:899–906. PMID:
15958358.

84. Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012; (9):CD002752. PMID:
22972058.

85. Wever-Pinzon O, Drakos SG, Fang JC. Team-based care for advanced heart failure. Heart Fail Clin. 2015; 11:467–477. PMID:
26142642.

86. Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002; 39:471–480. PMID:
11823086.

87. Morton G, Masters J, Cowburn PJ. Multidisciplinary team approach to heart failure management. Heart. 2018; 104:1376–1382. PMID:
29170356.

88. Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019; 73:1430–1443. PMID:
30922474.
89. Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018; 20:1735–1743. PMID:
30255969.

90. Ueno K, Kaneko H, Itoh H, et al. Effectiveness and approach of rehabilitation in patients with acute heart failure: a review. Korean Circ J. 2022; 52:576–592. PMID:
35929052.

91. Chun KH, Kang SM. Cardiac rehabilitation in heart failure. Int J Heart Fail. 2020; 3:1–14. PMID:
36263110.

92. Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015; 8:33–40. PMID:
25399909.

93. Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019; 7:691–705. PMID:
31302050.
94. Kim WS. Exercise-based cardiac rehabilitation in heart failure. Ann Cardiopulm Rehabil. 2021; 1:57–65.

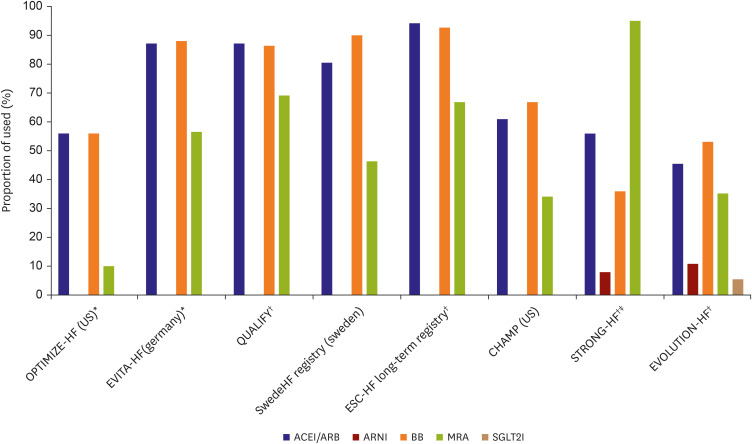
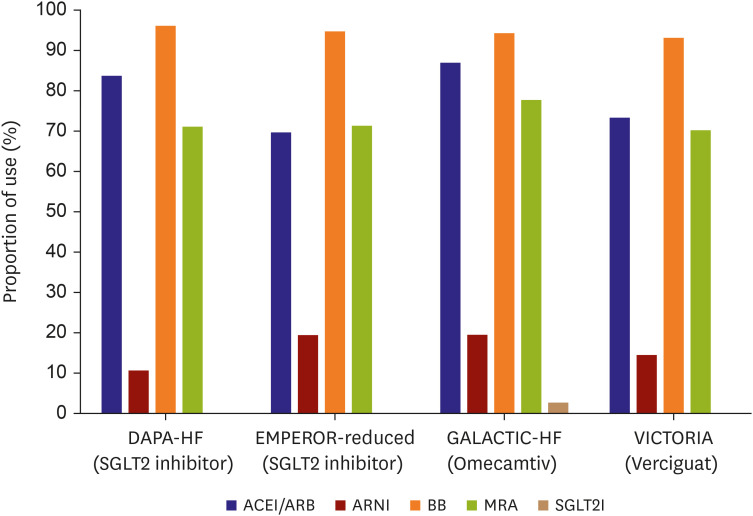
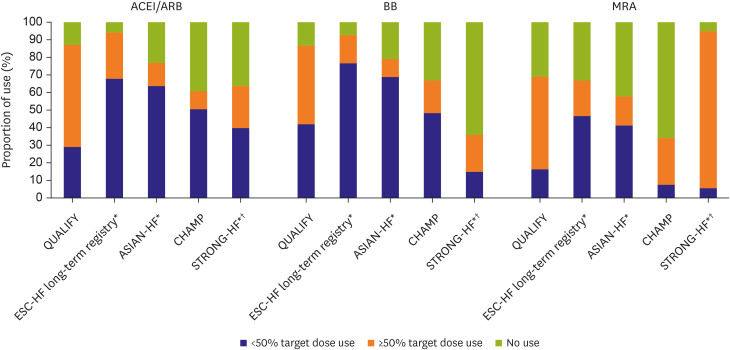
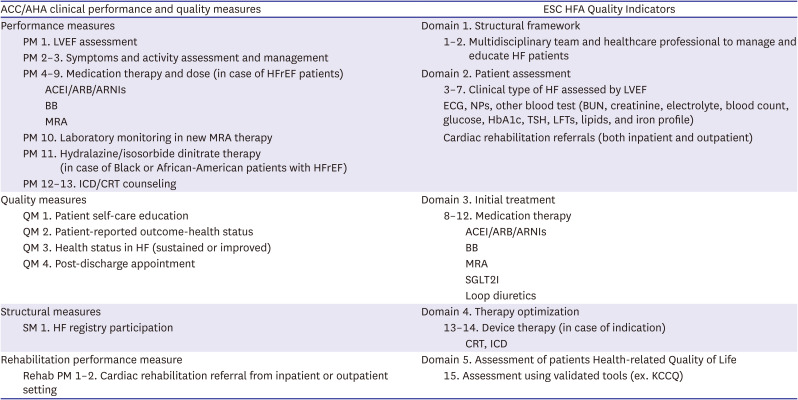





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download