1. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021; 42:373–498. PMID:
32860505.
2. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016; 374:2235–2245. PMID:
27042964.

3. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020; 383:1305–1316. PMID:
32865375.

4. Andrade JG, Deyell MW, Macle L, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023; 388:105–116. PMID:
36342178.

5. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021; 384:316–324. PMID:
33197158.

6. Taghji P, El Haddad M, Phlips T, et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol. 2018; 4:99–108. PMID:
29600792.

7. Straube F, Dorwarth U, Vogt J, et al. Differences of two cryoballoon generations: insights from the prospective multicentre, multinational FREEZE Cohort Substudy. Europace. 2014; 16:1434–1442. PMID:
24994074.

8. Ha FJ, Han HC, Sanders P, et al. Prevalence and prevention of oesophageal injury during atrial fibrillation ablation: a systematic review and meta-analysis. Europace. 2019; 21:80–90. PMID:
29912306.

9. Heeger CH, Sohns C, Pott A, et al. Phrenic nerve injury during cryoballoon-based pulmonary vein isolation: results of the worldwide YETI registry. Circ Arrhythm Electrophysiol. 2022; 15:e010516. PMID:
34962134.

10. Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006; 47:2498–2503. PMID:
16781380.
11. Mörtsell D, Arbelo E, Dagres N, et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace. 2019; 21:581–589. PMID:
30376055.

12. Kuck K, Brugada J, Albenque J. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016; 375:1100–1101.

13. Yokoyama K, Nakagawa H, Wittkampf FH, Pitha JV, Lazzara R, Jackman WM. Comparison of electrode cooling between internal and open irrigation in radiofrequency ablation lesion depth and incidence of thrombus and steam pop. Circulation. 2006; 113:11–19. PMID:
16380552.

14. Verma N, Gillespie CT, Argento AC, et al. Bronchial effects of cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2017; 14:12–16. PMID:
28007093.

15. Reddy VY, Neuzil P, Koruth JS, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019; 74:315–326. PMID:
31085321.

16. Lavee J, Onik G, Mikus P, Rubinsky B. A novel nonthermal energy source for surgical epicardial atrial ablation: irreversible electroporation. Heart Surg Forum. 2007; 10:E162–E167. PMID:
17597044.

17. Hong J, Stewart MT, Cheek DS, Francischelli DE, Kirchhof N. Cardiac ablation via electroporation. Annu Int Conf IEEE Eng Med Biol Soc. 2009; 2009:3381–3384. PMID:
19963798.
18. Buist TJ, Groen MH, Wittkampf FH, et al. Feasibility of Linear Irreversible Electroporation Ablation in the Coronary Sinus. Cardiovasc Eng Technol. 2023; 14:60–66. PMID:
35710861.

19. Koruth JS, Kuroki K, Iwasawa J, et al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace. 2020; 22:434–439. PMID:
31876913.

20. Gunawardene MA, Schaeffer BN, Jularic M, et al. Pulsed-field ablation combined with ultrahigh-density mapping in patients undergoing catheter ablation for atrial fibrillation: practical and electrophysiological considerations. J Cardiovasc Electrophysiol. 2022; 33:345–356. PMID:
34978360.

21. Gunawardene MA, Schaeffer BN, Jularic M, et al. Pulsed field ablation in patients with complex consecutive atrial tachycardia in conjunction with ultra-high density mapping: proof of concept. J Cardiovasc Electrophysiol. 2022; 33:2431–2443. PMID:
36259717.

22. Kueffer T, Seiler J, Madaffari A, et al. Pulsed-field ablation for the treatment of left atrial reentry tachycardia. J Interv Card Electrophysiol. 2022; [Epub ahead of print].

23. Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014; 16:295–320. PMID:
24905876.

24. van Driel VJ, Neven KG, van Wessel H, et al. Pulmonary vein stenosis after catheter ablation: electroporation versus radiofrequency. Circ Arrhythm Electrophysiol. 2014; 7:734–738. PMID:
24958397.
25. Witt CM, Sugrue A, Padmanabhan D, et al. Intrapulmonary vein ablation without stenosis: a novel balloon-based direct current electroporation approach. J Am Heart Assoc. 2018; 7:e009575. PMID:
29987121.

26. Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021; 7:614–627. PMID:
33933412.
27. Ekanem E, Reddy VY, Schmidt B, et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace. 2022; 24:1256–1266. PMID:
35647644.

28. Miklavčič D. Network for development of electroporation-based technologies and treatments: COST TD1104. J Membr Biol. 2012; 245:591–598. PMID:
22922776.

29. Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982; 1:841–845. PMID:
6329708.

30. Mir LM, Belehradek M, Domenge C, et al. Electrochemotherapy, a new antitumor treatment: first clinical trial. C R Acad Sci III. 1991; 313:613–618. PMID:
1723647.
31. Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008; 26:5896–5903. PMID:
19029422.

32. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005; 33:223–231. PMID:
15771276.

33. Scheinman MM, Morady F, Hess DS, Gonzalez R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982; 248:851–855. PMID:
7097946.

34. Gallagher JJ, Svenson RH, Kasell JH, et al. Catheter technique for closed-chest ablation of the atrioventricular conduction system. N Engl J Med. 1982; 306:194–200. PMID:
7054682.

35. Olgin JE, Scheinman MM. Comparison of high energy direct current and radiofrequency catheter ablation of the atrioventricular junction. J Am Coll Cardiol. 1993; 21:557–564. PMID:
8436734.

36. Kotnik T, Rems L, Tarek M, Miklavčič D. membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019; 48:63–91. PMID:
30786231.
37. Batista Napotnik T, Polajžer T, Miklavčič D. Cell death due to electroporation - a review. Bioelectrochemistry. 2021; 141:107871. PMID:
34147013.

38. Miklavcic D, Beravs K, Semrov D, Cemazar M, Demsar F, Sersa G. The importance of electric field distribution for effective in vivo electroporation of tissues. Biophys J. 1998; 74:2152–2158. PMID:
9591642.

39. Lee RC, Zhang D, Hannig J. Biophysical injury mechanisms in electrical shock trauma. Annu Rev Biomed Eng. 2000; 2:477–509. PMID:
11701521.

40. Bhatt DL, Gaylor DC, Lee RC. Rhabdomyolysis due to pulsed electric fields. Plast Reconstr Surg. 1990; 86:1–11. PMID:
2359775.

41. van Es R, Groen MH, Stehouwer M, Doevendans PA, Wittkampf FH, Neven K. In vitro analysis of the origin and characteristics of gaseous microemboli during catheter electroporation ablation. J Cardiovasc Electrophysiol. 2019; 30:2071–2079. PMID:
31347222.

42. Groen MH, van Es R, van Klarenbosch BR, et al. In vivo analysis of the origin and characteristics of gaseous microemboli during catheter-mediated irreversible electroporation. Europace. 2021; 23:139–146. PMID:
33111141.

43. Neven K, Füting A, Byrd I, et al. Absence of (sub-)acute cerebral events or lesions after electroporation ablation in the left-sided canine heart. Heart Rhythm. 2021; 18:1004–1011. PMID:
33617997.

44. Caluori G, Odehnalova E, Jadczyk T, et al. AC pulsed field ablation is feasible and safe in atrial and ventricular settings: a proof-of-concept chronic animal study. Front Bioeng Biotechnol. 2020; 8:552357. PMID:
33344428.

45. Mercadal B, Arena CB, Davalos RV, Ivorra A. Avoiding nerve stimulation in irreversible electroporation: a numerical modeling study. Phys Med Biol. 2017; 62:8060–8079. PMID:
28901954.

46. Wandel A, Ben-David E, Ulusoy BS, et al. Optimizing irreversible electroporation ablation with a bipolar electrode. J Vasc Interv Radiol. 2016; 27:1441–1450.e2. PMID:
27475242.

47. Cvetkoska A, Maček-Lebar A, Trdina P, Miklavčič D, Reberšek M. Muscle contractions and pain sensation accompanying high-frequency electroporation pulses. Sci Rep. 2022; 12:8019. PMID:
35577873.

48. Zager Y, Kain D, Landa N, Leor J, Maor E. Optimization of irreversible electroporation protocols for in-vivo myocardial decellularization. PLoS One. 2016; 11:e0165475. PMID:
27893744.

49. Weaver JC, Smith KC, Esser AT, Son RS, Gowrishankar TR. A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry. 2012; 87:236–243. PMID:
22475953.

50. Scuderi M, Dermol-Černe J, Amaral da Silva C, Muralidharan A, Boukany PE, Rems L. Models of electroporation and the associated transmembrane molecular transport should be revisited. Bioelectrochemistry. 2022; 147:108216. PMID:
35932533.

51. Kotnik T, Pucihar G, Rebersek M, Miklavcic D, Mir LM. Role of pulse shape in cell membrane electropermeabilization. Biochim Biophys Acta. 2003; 1614:193–200. PMID:
12896812.

52. Ben-David E, Ahmed M, Faroja M, et al. Irreversible electroporation: treatment effect is susceptible to local environment and tissue properties. Radiology. 2013; 269:738–747. PMID:
23847254.

53. Dermol-Černe J, Batista Napotnik T, Reberšek M, Miklavčič D. Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field. Sci Rep. 2020; 10:9149. PMID:
32499601.

54. Li W, Fan Q, Ji Z, Qiu X, Li Z. The effects of irreversible electroporation (IRE) on nerves. PLoS One. 2011; 6:e18831. PMID:
21533143.

55. Maor E, Ivorra A, Rubinsky B. Non thermal irreversible electroporation: novel technology for vascular smooth muscle cells ablation. PLoS One. 2009; 4:e4757. PMID:
19270746.

56. Sugrue A, Vaidya V, Witt C, et al. Irreversible electroporation for catheter-based cardiac ablation: a systematic review of the preclinical experience. J Interv Card Electrophysiol. 2019; 55:251–265. PMID:
31270656.

57. Kaminska I, Kotulska M, Stecka A, et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen Physiol Biophys. 2012; 31:19–25. PMID:
22447827.

58. Hunter DW, Kostecki G, Fish JM, Jensen JA, Tandri H. In vitro cell selectivity of reversible and irreversible: electroporation in cardiac tissue. Circ Arrhythm Electrophysiol. 2021; 14:e008817. PMID:
33729827.
59. Baena-Montes JM, O’Halloran T, Clarke C, et al. Electroporation parameters for human cardiomyocyte ablation in vitro. J Cardiovasc Dev Dis. 2022; 9:240. PMID:
36005404.

60. Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015; 62:4–20. PMID:
25389236.

61. Wijffels MC, Timmermans CC, van Suylen RJ, Rodriguez LM. Internal atrial shock delivery by standard diagnostic electrophysiology catheters in goats: effects on atrial electrogram amplitude and tissue architecture. Europace. 2007; 9:203–207. PMID:
17350984.

62. Wittkampf FH, van Driel VJ, van Wessel H, et al. Feasibility of electroporation for the creation of pulmonary vein ostial lesions. J Cardiovasc Electrophysiol. 2011; 22:302–309. PMID:
20653809.

63. Neven K, van Es R, van Driel V, et al. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol. 2017; 10:e004672. PMID:
28487347.

64. van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015; 12:1838–1844. PMID:
25998897.

65. Howard B, Haines DE, Verma A, et al. Characterization of phrenic nerve response to pulsed field ablation. Circ Arrhythm Electrophysiol. 2022; 15:e010127. PMID:
35649121.

66. Neven K, van Driel V, van Wessel H, et al. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ Arrhythm Electrophysiol. 2014; 7:913–919. PMID:
25156260.

67. Wittkampf FH, van Driel VJ, van Wessel H, et al. Myocardial lesion depth with circular electroporation ablation. Circ Arrhythm Electrophysiol. 2012; 5:581–586. PMID:
22492429.

68. Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F. Epicardial linear electroporation ablation and lesion size. Heart Rhythm. 2014; 11:1465–1470. PMID:
24768609.

69. Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F. Myocardial lesion size after epicardial electroporation catheter ablation after subxiphoid puncture. Circ Arrhythm Electrophysiol. 2014; 7:728–733. PMID:
25015945.

70. Reddy VY, Koruth J, Jais P, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018; 4:987–995. PMID:
30139499.
71. Reddy VY, Anic A, Koruth J, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020; 76:1068–1080. PMID:
32854842.

72. Kawamura I, Neuzil P, Shivamurthy P, et al. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm. 2021; 18:878–884. PMID:
33647464.

73. Verma A, Neal R, Evans J, et al. Characteristics of pulsed electric field cardiac ablation porcine treatment zones with a focal catheter. J Cardiovasc Electrophysiol. 2023; 34:99–107. PMID:
36335638.

74. Verma A, Boersma L, Haines DE, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol. 2022; 15:e010168. PMID:
34964367.

75. Yavin H, Brem E, Zilberman I, et al. Circular multielectrode pulsed field ablation catheter lasso pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021; 14:e009229. PMID:
33417475.

76. Anter E, Neužil P, Rackauskas G, et al. A lattice-tip temperature-controlled radiofrequency ablation catheter for wide thermal lesions: first-in-human experience with atrial fibrillation. JACC Clin Electrophysiol. 2020; 6:507–519. PMID:
32439034.

77. Reddy VY, Anter E, Rackauskas G, et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol. 2020; 13:e008718. PMID:
32383391.

78. Younis A, Zilberman I, Krywanczyk A, et al. Effect of pulsed-field and radiofrequency ablation on heterogeneous ventricular scar in a swine model of healed myocardial infarction. Circ Arrhythm Electrophysiol. 2022; 15:e011209. PMID:
36194542.

79. Yavin HD, Higuchi K, Younis A, Anter E. Lattice-tip catheter for single-shot pulmonary vein isolation with pulsed field ablation. J Interv Card Electrophysiol. 2022; [Epub ahead of print].

80. Verma A, Feld GK, Cox JL, et al. Combined pulsed field ablation with ultra-low temperature cryoablation: a preclinical experience. J Cardiovasc Electrophysiol. 2022; 1–10.

81. Loh P, van Es R, Groen MH, et al. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2020; 13:e008192. PMID:
32898450.

82. Avazzadeh S, McBride S, O’Brien B, et al. Ganglionated plexi ablation for the treatment of atrial fibrillation. J Clin Med. 2020; 9:3081. PMID:
32987820.

83. Madhavan M, Venkatachalam KL, Swale MJ, et al. Novel percutaneous epicardial autonomic modulation in the canine for atrial fibrillation: results of an efficacy and safety study. Pacing Clin Electrophysiol. 2016; 39:407–417. PMID:
26854009.

84. Padmanabhan D, Naksuk N, Killu AK, et al. Electroporation of epicardial autonomic ganglia: safety and efficacy in medium-term canine models. J Cardiovasc Electrophysiol. 2019; 30:607–615. PMID:
30680839.

85. Tohoku S, Chun KR, Bordignon S, et al. Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. Europace. 2023; 25:433–440. PMID:
36427201.

86. Kawamura I, Neuzil P, Shivamurthy P, et al. How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace. 2021; 23:1757–1766. PMID:
34151947.

87. Nakatani Y, Sridi-Cheniti S, Cheniti G, et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace. 2021; 23:1767–1776. PMID:
34240134.

88. Füting A, Reinsch N, Höwel D, Brokkaar L, Rahe G, Neven K. First experience with pulsed field ablation as routine treatment for paroxysmal atrial fibrillation. Europace. 2022; 24:1084–1092. PMID:
35513354.

89. Avazzadeh S, O’Brien B, Coffey K, O’Halloran M, Keane D, Quinlan LR. Establishing irreversible electroporation electric field potential threshold in a suspension in vitro model for cardiac and neuronal cells. J Clin Med. 2021; 10:5443. PMID:
34830725.

90. Schmidt B, Bordignon S, Tohoku S, et al. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022; 15:e010817. PMID:
35617232.

91. Lemoine MD, Fink T, Mencke C, et al. Pulsed-field ablation-based pulmonary vein isolation: acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin Res Cardiol. 2022; [Epub ahead of print].

92. Kuroki K, Whang W, Eggert C, et al. Ostial dimensional changes after pulmonary vein isolation: pulsed field ablation vs radiofrequency ablation. Heart Rhythm. 2020; 17:1528–1535. PMID:
32380290.

93. Cochet H, Nakatani Y, Sridi-Cheniti S, et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021; 23:1391–1399. PMID:
33961027.

94. Reddy VY, Petru J, Funasako M, et al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation. 2022; 146:1808–1819. PMID:
36134574.

95. Della Rocca DG, Del Monte A, Bala G, et al. Transient inferior ST-segment elevation and ventricular fibrillation after cavotricuspid isthmus pulsed-field ablation. JACC Clin Electrophysiol. 2023; [Epub ahead of print].

96. Reinsch N, Füting A, Höwel D, Bell J, Lin Y, Neven K. Cerebral safety after pulsed field ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2022; 19:S1547-5271(22)02090-2.

97. Chen S, Chun JK, Bordignon S, et al. Pulsed field ablation-based pulmonary vein isolation in atrial fibrillation patients with cardiac implantable electronic devices: practical approach and device interrogation (PFA in CIEDs). J Interv Card Electrophysiol. 2022; [Epub ahead of print].

98. Urbanek L, Chen S, Bordignon S, et al. First pulse field ablation of an incessant atrial tachycardia from the right atrial appendage. J Interv Card Electrophysiol. 2022; 65:577–578. PMID:
36029430.

99. Adeliño R, Combes S, El Bouazzaoui R, Albenque JP, Combes N, Boveda S. Pulsed-field ablation of recurrent right atrial tachycardia: expanding the use of electroporation beyond atrial fibrillation. Europace. 2022; euac198.

100. du Pré BC, van Driel VJ, van Wessel H, et al. Minimal coronary artery damage by myocardial electroporation ablation. Europace. 2013; 15:144–149. PMID:
22654094.
101. Balantič K, Miklavčič D, Križaj I, Kramar P. The good and the bad of cell membrane electroporation. Acta Chim Slov. 2021; 68:753–764. PMID:
34918751.

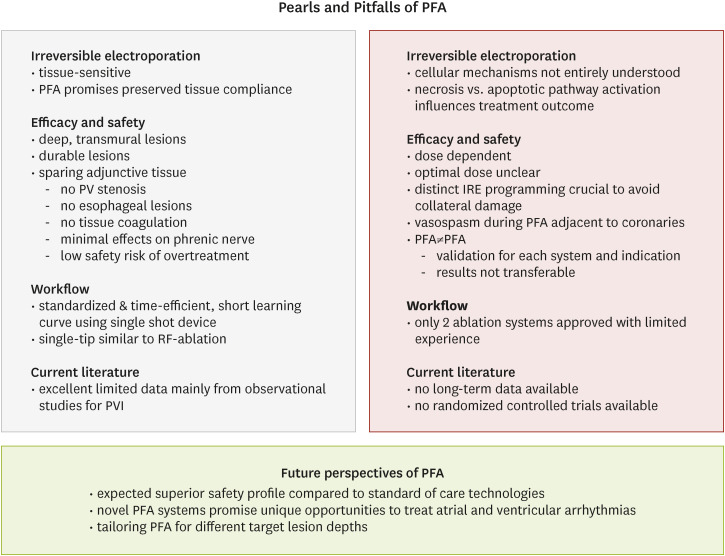
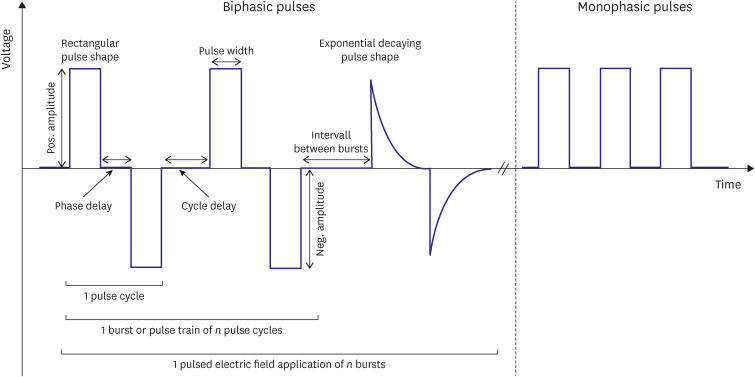
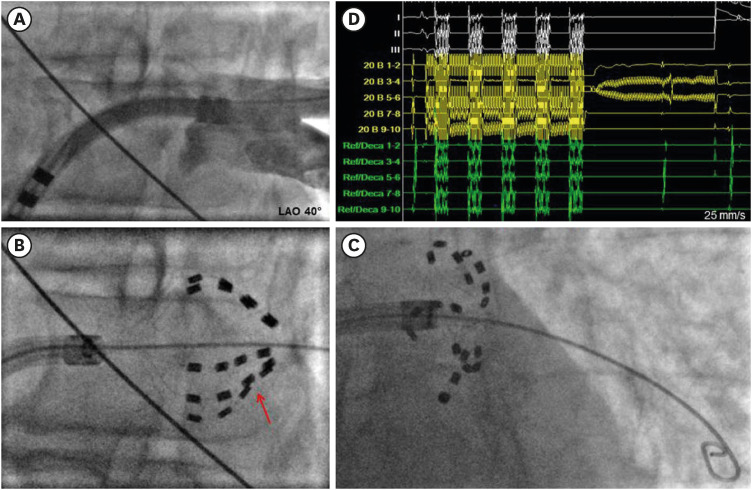
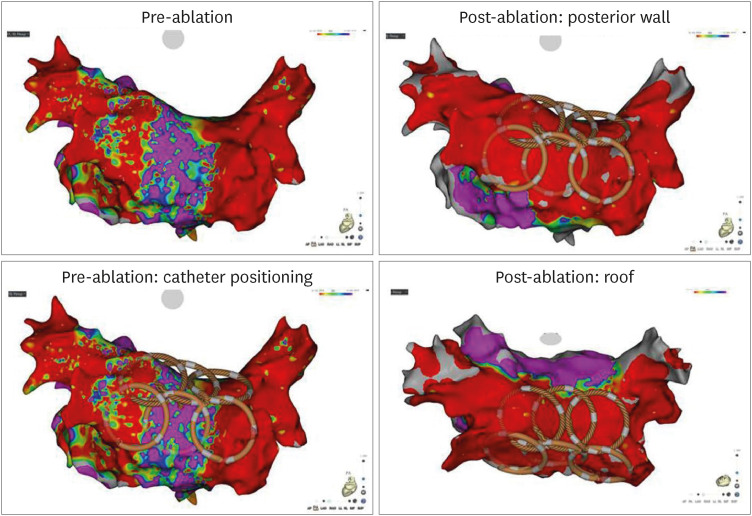




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download