1. Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019; 1:4–24. PMID:
36262736.

2. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.

3. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 79:1757–1780. PMID:
35379504.
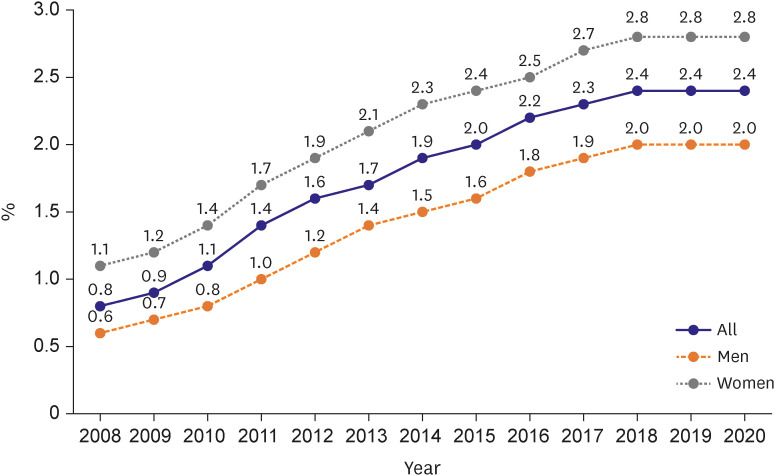
4. Cho DH, Yoo BS. Current prevalence, incidence, and outcomes of heart failure with preserved ejection fraction. Heart Fail Clin. 2021; 17:315–326. PMID:
34051964.

5. Shim CY. Heart failure with preserved ejection fraction: the major unmet need in cardiology. Korean Circ J. 2020; 50:1051–1061. PMID:
33150751.

6. Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016; 37:455–462. PMID:
26374849.

7. Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381:1609–1620. PMID:
31475794.

8. Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018; 20:1230–1239. PMID:
29431256.

9. Lam CS, Solomon SD. Delivering therapeutic efficacy across the ejection fraction spectrum of heart failure. Circulation. 2022; 146:1193–1195. PMID:
36029466.

10. Cho JH, Choe WS, Cho HJ, et al. Comparison of characteristics and 3-year outcomes in patients with acute heart failure with preserved, mid-range, and reduced ejection fraction. Circ J. 2019; 83:347–356. PMID:
30404976.

11. Arrigo M, Huber LC, Winnik S, et al. Right ventricular failure: pathophysiology, diagnosis and treatment. Card Fail Rev. 2019; 5:140–146. PMID:
31768270.

12. Lee HH, Cho SM, Lee H, et al. Korea heart disease fact sheet 2020: analysis of nationwide data. Korean Circ J. 2021; 51:495–503. PMID:
34085422.

13. Kang SM. Key role of the Korean Society of Heart Failure: moving towards a global and individualized approach. Int J Heart Fail. 2022; 4:136–138. PMID:
36262798.

14. Yingchoncharoen T, Wu TC, Choi DJ, Ong TK, Liew HB, Cho MC. Economic burden of heart failure in Asian countries with different healthcare systems. Korean Circ J. 2021; 51:681–693. PMID:
34227265.

15. Chung H, Sohn IS. Economic burden of heart failure in Asian countries based on real-world data. Korean Circ J. 2021; 51:694–695. PMID:
34227271.

16. Cho SM, Lee H, Kim HC. Sex- and age-specific trends in cardiovascular health in Korea, 2007–2018. Korean Circ J. 2021; 51:922–935. PMID:
34719898.

17. Cho DH, Lee CJ, Son JW, Choi J, Hwang J, Yoo BS. Temporal trends in heart failure over 11 years in the aging Korean population: a retrospective study using the national health insurance database. PLoS One. 2022; 17:e0279541. PMID:
36576935.

18. Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000; 139:72–77. PMID:
10618565.

19. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28–292. PMID:
24352519.
20. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6:606–619. PMID:
23616602.
21. Park JJ, Lee CJ, Park SJ, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021; 3:224–236. PMID:
36262554.

22. Kim MS. The long journey to obtaining the epidemiological data of heart failure in Korea. Int J Heart Fail. 2021; 3:221–223. PMID:
36262559.

23. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002; 106:3068–3072. PMID:
12473553.

24. Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016; 46:658–664. PMID:
27721857.

25. Choi DJ, Han S, Jeon ES, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean Heart Failure Registry. Korean Circ J. 2011; 41:363–371. PMID:
21860637.

26. Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017; 47:341–353. PMID:
28567084.

27. Lee HY, Oh BH. Paradigm shifts of heart failure therapy: do we need another paradigm? Int J Heart Fail. 2020; 2:145–156. PMID:
36262366.

28. Mant J, Doust J, Roalfe A, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. 2009; 13:1–207. iii

29. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011; 124:2865–2873. PMID:
22104551.

30. Lee KS, Noh J, Park SM, et al. Evaluation and management of patients with diabetes and heart failure: a Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement. Int J Heart Fail. 2023; 5:1–20. PMID:
36818141.

31. Gohar A, Rutten FH, den Ruijter H, et al. Mid-regional pro-atrial natriuretic peptide for the early detection of non-acute heart failure. Eur J Heart Fail. 2019; 21:1219–1227. PMID:
31209992.

32. Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017; 18:1301–1310. PMID:
29045589.

33. Wettersten N. Biomarkers in acute heart failure: diagnosis, prognosis, and treatment. Int J Heart Fail. 2021; 3:81–105. PMID:
36262882.

34. Schellenberger U, O’Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006; 451:160–166. PMID:
16750161.

35. Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today Ther Strateg. 2012; 9:e131–e139.

36. Myhre PL, Vaduganathan M, Claggett B, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019; 73:1264–1272. PMID:
30846338.
37. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013; 310:66–74. PMID:
23821090.

38. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013; 62:1365–1372. PMID:
23810874.

39. Zaphiriou A, Robb S, Murray-Thomas T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail. 2005; 7:537–541. PMID:
15921792.

40. Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW. Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. J Card Fail. 2011; 17:729–734. PMID:
21872142.

41. Januzzi JL Jr, Chen-Tournoux AA, Christenson RH, et al. N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. J Am Coll Cardiol. 2018; 71:1191–1200. PMID:
29544601.
42. Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010; 55:2062–2076. PMID:
20447528.

43. Yoo BS, Kim WJ, Jung HS, et al. The clinical experiences of B-type natriuretic peptide blood concentrations for diagnosis in congestive heart failure: the single hospital experience based on the large clinical database. Korean Circ J. 2004; 34:684–692.

44. Maisel A, Mueller C, Adams K Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008; 10:824–839. PMID:
18760965.

45. Taub PR, Daniels LB, Maisel AS. Usefulness of B-type natriuretic peptide levels in predicting hemodynamic and clinical decompensation. Heart Fail Clin. 2009; 5:169–175. PMID:
19249686.

46. Anand IS, Fisher LD, Chiang YT, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2003; 107:1278–1283. PMID:
12628948.

47. Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012; 156:767–775. W-261W-262PMID:
22665814.

48. Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004; 43:635–641. PMID:
14975475.

49. Kang SH, Park JJ, Choi DJ, et al. Prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart. 2015; 101:1881–1888. PMID:
26319121.

50. Zile MR, Claggett BL, Prescott MF, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016; 68:2425–2436. PMID:
27908347.

51. Weiner RB, Baggish AL, Chen-Tournoux A, et al. Improvement in structural and functional echocardiographic parameters during chronic heart failure therapy guided by natriuretic peptides: mechanistic insights from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study. Eur J Heart Fail. 2013; 15:342–351. PMID:
23132825.

52. Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017; 318:713–720. PMID:
28829876.

53. Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009; 15:392–397. PMID:
19633546.

54. Fonarow GC, Peacock WF, Horwich TB, et al. Usefulness of B-type natriuretic peptide and cardiac troponin levels to predict in-hospital mortality from ADHERE. Am J Cardiol. 2008; 101:231–237. PMID:
18178412.

55. Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020; 22:350–361. PMID:
31721381.

56. Lee J, Kim EJ. ST2 as a biomarker to show the preventive effect of exercise in myocardial injury by doxorubicin? Int J Heart Fail. 2021; 3:117–120. PMID:
36262878.

57. Hu SS, Kong LZ, Gao RL, et al. Outline of the report on cardiovascular disease in China, 2010. Biomed Environ Sci. 2012; 25:251–256. PMID:
22840574.
58. Aimo A, Vergaro G, Passino C, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017; 5:280–286. PMID:
27816512.

59. van Kimmenade RR, Januzzi JL Jr, Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006; 48:1217–1224. PMID:
16979009.

60. Imran TF, Shin HJ, Mathenge N, et al. Meta-analysis of the usefulness of plasma galectin-3 to predict the risk of mortality in patients with heart failure and in the general population. Am J Cardiol. 2017; 119:57–64. PMID:
28247849.

61. Park JJ, Choi DJ, Yoon CH, et al. Prognostic value of C-reactive protein as an inflammatory and N-terminal probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry). Am J Cardiol. 2014; 113:511–517. PMID:
24315115.

62. Zairis MN, Tsiaousis GZ, Georgilas AT, et al. Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int J Cardiol. 2010; 141:284–290. PMID:
19157603.

63. Cho DH, Son JW, Lee CJ, et al. Prognostic value of short-term follow-up of multiple biomarkers after discharge in hospitalized patients with acute heart failure (POSTBIO-HF): rationale and study design. Int J Heart Fail. 2022; 4:110–116. PMID:
36263107.

64. Anand IS, Kempf T, Rector TS, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010; 122:1387–1395. PMID:
20855664.

65. Bouabdallaoui N, Claggett B, Zile MR, et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2018; 20:1701–1709. PMID:
30204280.

66. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1–39.e14. PMID:
25559473.

67. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018; 71:1947–1957. PMID:
29724346.

68. Yoon YE, Hong YJ, Kim HK, et al. 2014 Korean guidelines for appropriate utilization of cardiovascular magnetic resonance imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean Circ J. 2014; 44:359–385. PMID:
25469139.

69. Sugeng L, Mor-Avi V, Weinert L, et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010; 3:10–18. PMID:
20129525.

70. Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement--executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur Heart J. 2009; 30:278–289. PMID:
19001473.

71. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41:407–477. PMID:
31504439.
72. Valle-Muñoz A, Estornell-Erill J, Soriano-Navarro CJ, et al. Late gadolinium enhancement-cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure. Eur J Echocardiogr. 2009; 10:968–974. PMID:
19755468.

73. Hamilton-Craig C, Strugnell WE, Raffel OC, Porto I, Walters DL, Slaughter RE. CT angiography with cardiac MRI: non-invasive functional and anatomical assessment for the etiology in newly diagnosed heart failure. Int J Cardiovasc Imaging. 2012; 28:1111–1122. PMID:
21789747.

74. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005; 26:1461–1474. PMID:
15831557.

75. Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 40:2156–2164. PMID:
12505229.

76. Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009; 120:1969–1977. PMID:
19884472.

77. Gao P, Yee R, Gula L, et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012; 5:448–456. PMID:
22572740.

78. Puntmann VO, Carr-White G, Jabbour A, et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging. 2016; 9:40–50. PMID:
26762873.
79. Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018; 72:3158–3176. PMID:
30545455.

80. Seferović PM, Polovina M, Bauersachs J, et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019; 21:553–576. PMID:
30989768.
81. Authors/Task Force members. Elliott PM, Anastasakis A, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014; 35:2733–2779. PMID:
25173338.

82. Lee HY, Jeon ES, Kang SM, Kim JJ. Initial report of the Korean Organ Transplant Registry (KOTRY): heart transplantation. Korean Circ J. 2017; 47:868–876. PMID:
29035428.

83. Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy-a Heart Failure Society of America practice guideline. J Card Fail. 2018; 24:281–302. PMID:
29567486.

84. Park HY. Hereditary dilated cardiomyopathy: recent advances in genetic diagnostics. Korean Circ J. 2017; 47:291–298. PMID:
28567076.

85. Tayal U, Ware JS, Lakdawala NK, Heymans S, Prasad SK. Understanding the genetics of adult-onset dilated cardiomyopathy: what a clinician needs to know. Eur Heart J. 2021; 42:2384–2396. PMID:
34153989.

86. Yogasundaram H, Alhumaid W, Dzwiniel T, Christian S, Oudit GY. Cardiomyopathies and genetic testing in heart failure: role in defining phenotype-targeted approaches and management. Can J Cardiol. 2021; 37:547–559. PMID:
33493662.

87. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC): developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200. PMID:
27206819.

88. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017; 136:e137–e161. PMID:
28455343.

89. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017; 135:825–838. PMID:
28039229.

90. Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging. 2020; 13:272–282. PMID:
31202741.
91. Shim CY. Stress testing in heart failure with preserved ejection fraction. Heart Fail Clin. 2021; 17:435–445. PMID:
34051975.

92. Reddy YN, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018; 138:861–870. PMID:
29792299.

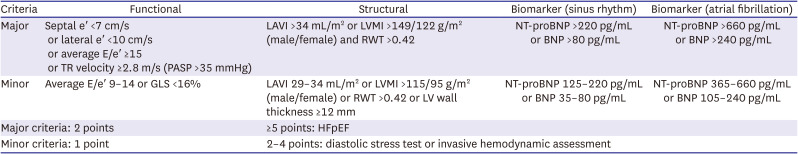
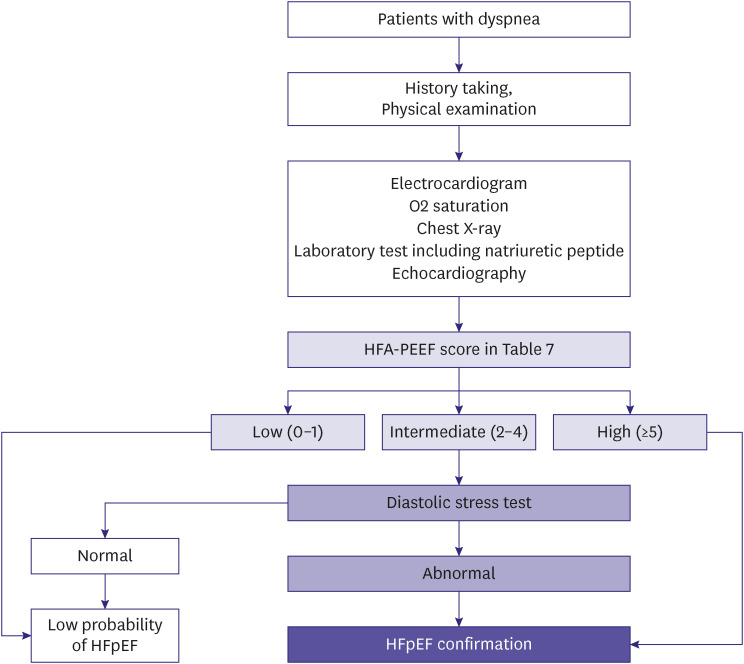
93. Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020; 22:391–412. PMID:
32133741.

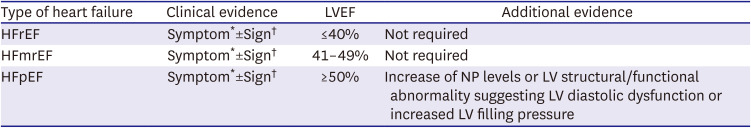
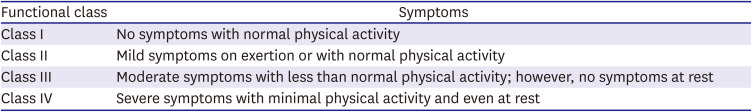
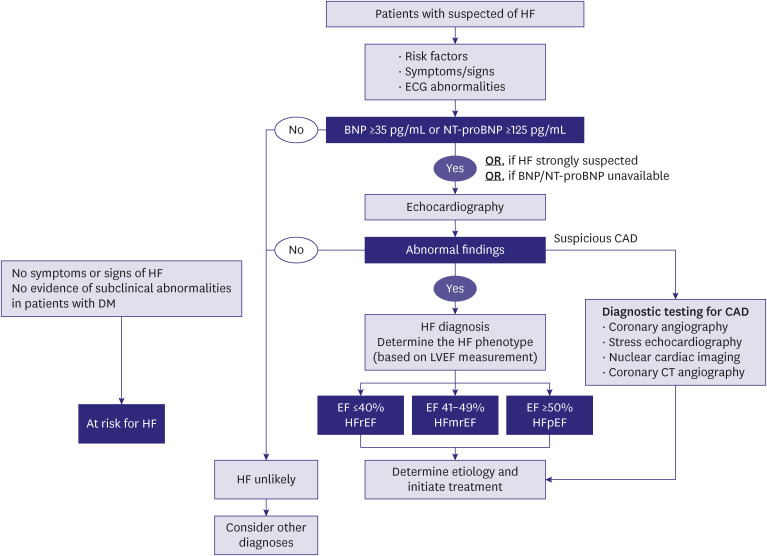
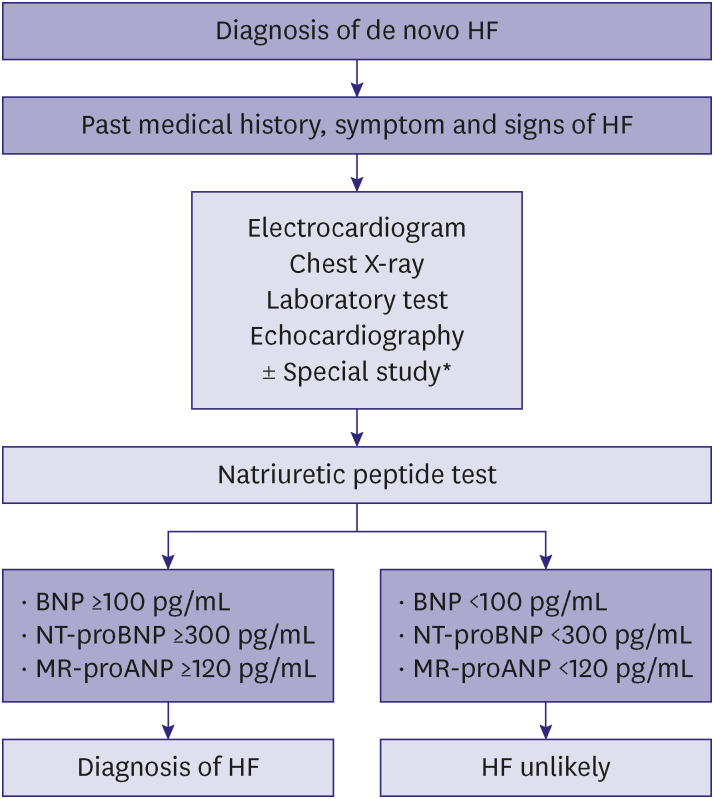




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download