INTRODUCTION
Brugada syndrome (BrS) is an inherited arrhythmia syndrome that can present as sudden cardiac death (SCD) through life-threatening ventricular tachyarrhythmia without the manifestations of structural heart disease.
1) The exact mechanism of BrS still remains unclear. However, one of the mechanisms has been suggested to be related to the uneven distribution of transient outward potassium current (
Ito) channels between the epicardium and endocardium, leading to the development of phase 2 reentry and, ultimately, ventricular arrhythmia.
2) The net outward shift of electrical current during the phase 1 period of epicardial action potentials (APs) is most prominent in the right ventricular outflow tract (RVOT).
3)4) As a result, it is assumed that Brugada pattern ECG appears in lead V1 and 2. According to current guidelines, an implantable cardioverter-defibrillator (ICD) is considered to be the first-line therapy.
5)6) However, an ICD might not be an adequate solution for very young patients, and there might be regions where ICDs are not available owing to financial issues. Furthermore, the long-term durability of ICD leads and frequent appropriate or inappropriate shocks are also problematic. Therefore, pharmacologic complementary treatments might be needed for reducing fatal arrhythmic events in patients with BrS.
Artemisinin is derived from an herbal plant that has been previously commonly used as an antipyretic in ancient China; nowadays, it is widely used as an antimalarial drug. In addition to its antimalarial effect, its antiarrhythmic effect, which includes suppression of the
IK1,
Ito, and
IKs channels, was reported previously.
7)8)9)10) Therefore, we evaluated the hypothesis that inhibition of
Ito channels by artemisinin may play a role in the suppression of ventricular arrhythmia in canine wedge preparation models of BrS.
METHODS
Ethical statement
All experiments were carried out according to the guidelines mentioned in the Guide for Care and Use of Laboratory Animals. The study was approved by the Animal Care and Use Committee at Chonnam National University, Gwangju, Republic of Korea (CNU IACUC-20044).
Wedge preparations and electrogram recordings
A detailed description of the arterially-perfused ventricular wedge preparation and recording of the transmembrane AP were previously reported.
11)12) Adult mongrel dogs (25 to 30 kg) of either sex were used. The animals were supplied by Orient Bio, Inc. (Seongnam, Korea). The dogs underwent anticoagulation with heparin (1,000 U/kg, intravenous [IV]) and were anesthetized with pentobarbital (30 to 35 mg/kg, IV). The chest was opened via a left thoracotomy and the heart was explanted. Then, the heart was placed in a cold cardioplegic solution (Tyrode’s solution containing 12 mmol/L KCl). Transmural wedge preparations were dissected (2.2×2.4×2.0 to 2.2×1.8×1.9 cm
3) from the right ventricular free wall. The preparations were cannulated into the marginal branch of the right coronary artery and perfused with a cardioplegic solution (Tyrode’s solution containing 12 mmol/L KCl). Unperfused tissue was meticulously removed using a razor blade. Then, preparations were placed in a tissue bath and perfused with oxygenated Tyrode’s solution (NaCl 129 mM, KCl 4 mM, NaH
2PO
3 20 mM, CaC1
2 1.8 mM, MgSO
4 0.5 mM, and glucose 5.5 mM; pH: 7.4). The perfusate was infused using a roller pump (Masterflex 7518-10; Cole-Parmer Instrument Co, Vernon Hills, IL, USA) at a constant flow rate of 8 to 10 mL/min. The temperature was maintained at 37±0.5°C.
The preparations were equilibrated until the tissue bath became electrically stable. The endocardial surface was stimulated at a basic cycle length of 1,000 ms with bipolar silver electrodes that were insulated except at the tips. Transmural pseudo-electrocardiograms (ECGs) were recorded using two electrodes, which consisted of AgCl half cells, placed 1 to 1.5 cm from the endocardial and epicardial surfaces in the tissue bath, along the same axis with the transmembrane recordings. The epicardial electrode was connected to the positive input of the ECG amplifier (MP150CE; Biopac Systems, Inc., Goleta, CA, USA).
Transmembrane APs were simultaneously recorded at the epicardial and endocardial sites, using floating microelectrodes (DC resistance: 10 to 20 MΩ) filled with 2.7 mol/L KCl. Both electrodes were connected to a high-input impedance amplifier (Electro 705 electrometer, FD223-G; World Precision Instruments Inc., Sarasota, FL, USA). Impalements were obtained from the epicardial and endocardial surfaces of the preparation at positions approximating the transmural axis of the pseudo-ECG recording (
Figure 1). Eight wedge preparations were made for artemisinin and 2 were made for time-control.
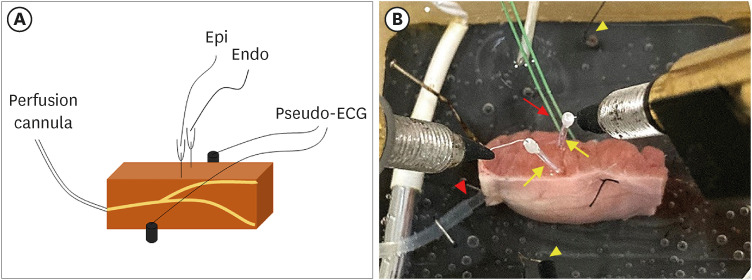
Figure 1
A right ventricular coronary-perfused preparation model. (A) Scheme of a right ventricular canine coronary-perfused preparation. Transmembrane APs are recorded simultaneously at the epicardial and endocardial sites with floating microelectrodes. A pseudo-ECG is recorded using AgCl electrodes placed across the bath, positioned transmurally along the midline of the preparation. (B) A right ventricle wedge preparation in a tissue bath. Pacing on the endocardial surface is depicted by the red arrow, and the floating microelectrodes on the endocardium and epicardium are highlighted by the yellow arrows. Two pseudo-ECG leads are placed approximately 1–1.5 cm from the endocardium and epicardium (yellow arrowheads). The coronary artery is perfused with oxygenated Tyrode’s solution (red arrowhead).
AP = action potential; Endo = endocardium; Epi = epicardium; ECG = electrocardiogram.
Measurement and calculations
The recording and analysis of the pseudo-ECGs and, APs were performed using Spike 2 software for Windows 10 (Cambridge Electronic Design Ltd., Cambridge, UK).
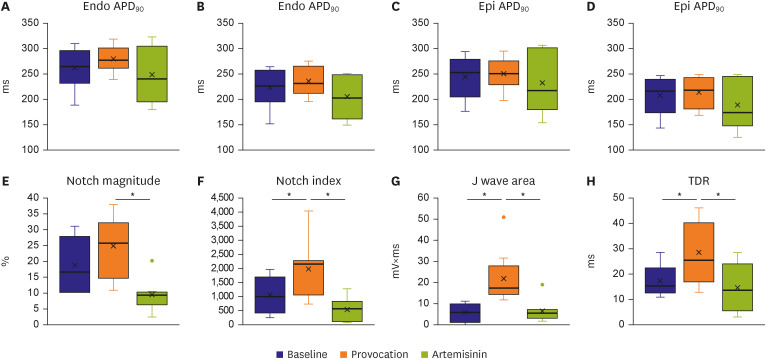
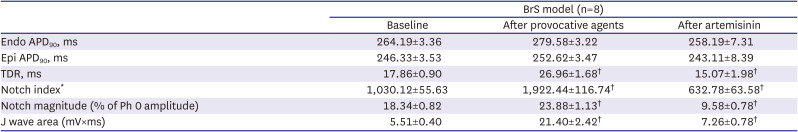
The starting point of the J wave was defined from pseudo-ECG signals. If the starting point of the J wave was clear, it was set to the time corresponding to the start of the notch between the R and J waves. In case this separation was not clear, the start time was set at the moment when the negative derivative was at its maximal value after the downslope of the R wave. The J wave area was calculated as mV×ms.
13)
The AP notch magnitude (phase 1 magnitude/phase 0 amplitude × 100) and notch index (notch magnitude × [phase 0 − phase 2 interval]) of the epicardium, which approximates the area of the notch, were measured in AP recordings (
Figure 2).
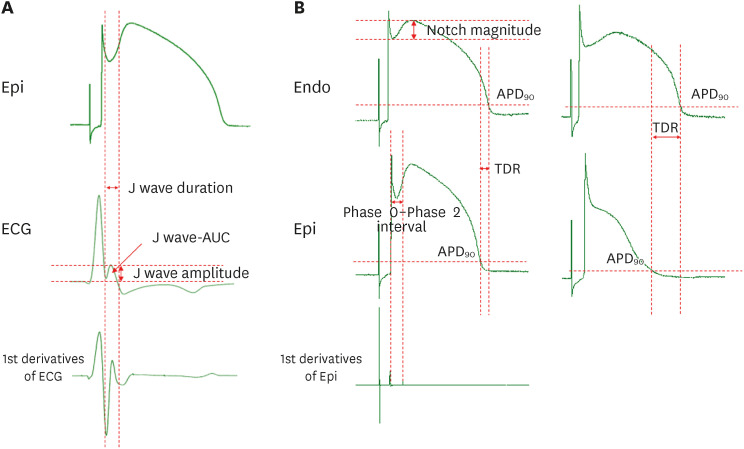
Figure 2
Measurement of AP. (A) Measurement of J wave parameters. (B) Measurement of AP parameters and transmural dispersion of repolarization when the AP dome is maintained or lost. The J wave area was calculated as mV×ms.
AP = action potential; APD90 = action potential duration at 90% repolarization; AUC = area under curve; ECG = electrocardiogram; Endo = endocardium; Epi = epicardium; TDR = transmural dispersion of repolarization.
Transmural dispersion of repolarization (TDR) was calculated as the longest interval between the endocardial and epicardial AP durations at 90% of repolarization (APD
90) values in simultaneously recorded APs, corrected by activation time (AT) differences as follows: TDR = (APD
90 endocardium + AT endocardium) + (APD
90 epicardium + AT epicardium) (
Figure 2).
Arrhythmia induction
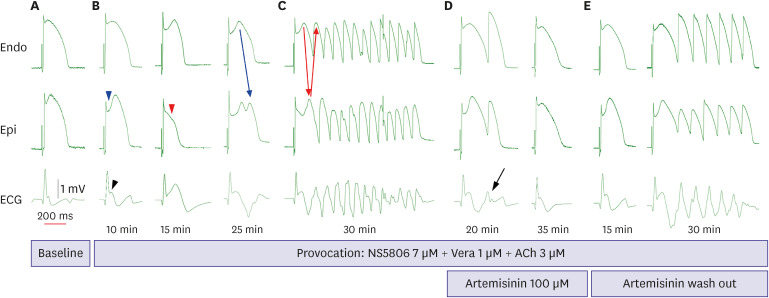
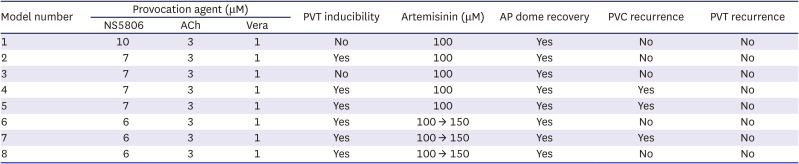
Acetylcholine (3 μM), calcium channel blocker verapamil (1 μM), and Ito channel agonist NS5806 (1-[2,4-dibromo-6-(1H-tetrazol-5-yl)-phenyl]-3-(3,5-bis-trifluoromethyl-phenyl)-urea; Sigma-Aldrich, St. Louis, MO, USA) (6–10 μM) were used to pharmacologically mimic BrS. Arrhythmia inducibility was defined as ventricular tachyarrhythmia developed after infusion of provocation agents during stable endocardial stimulation at a basic cycle length of 1,000 ms. Artemisinin (C15H22O5; Sigma-Aldrich) (100–150 μM) was then perfused to ameliorate the ventricular tachyarrhythmia in the BrS models. In the case of spontaneous intractable ventricular fibrillation before BrS induction, we assessed that the tissue has got failure and discarded the preparations.
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). The Mann–Whitney rank-sum test was used to compare the continuous variables as the sample size was small and had no normality. The Wilcoxon signed rank test was used to assess the effects of before and after artemisinin. A 2-sided p value of <0.05 was considered statistically significant. All statistical tests were performed using IBM SPSS version 27.0 (IBM Corp., Armonk, NY, USA).
DISCUSSION
The present study revealed that artemisinin suppressed the ECG manifestations, such as fatal ventricular arrhythmia, of BrS in a canine RV wedge preparation model. Our findings suggest that the suppressive effect of artemisinin on J waves and ventricular arrhythmia might relate to the inhibition of potassium channels, including
Ito channels. This effect was similar to that of other
Ito channel suppressing agents, such as quinidine.
14)
The depolarization and repolarization hypotheses of BrS set the stage for long debates over the underlying pathophysiology.
15) We admit that the depolarization abnormality resulting in a conduction delay may contribute to the pathogenesis of BrS.
16)17)18) However, our study adds one evidence to the repolarization hypothesis by showing the pivotal role of potassium currents. In the view of depolarization hypothesis, quinidine would be expected to accentuate conduction delay, because quinidine is also an inhibitor of sodium channel. However, quinidine is observed to suppress the ventricular storm in BrS and resolve BrS phenotype ECG, which is advocated by repolarization hypothesis.
4)19)20) Thus, we think it is not easy to explain the mechanism with a single hypothesis. Recently, Behr et al.
21) also stated that altered depolarization and secondary repolarization effects contribute together to the pathogenesis of BrS.
Artemisinin is an antimalarial drug with proven efficacy and is used worldwide. It is derived from the Chinese sweet wormwood plant
Artemisia annua. The ancient Chinese have used it as an herbal remedy for febrile symptoms for at least 2000 years.
22) Interestingly, some researchers reported that artemisinin has an antiarrhythmic effect by inhibiting multiple ion channels.
9)10) Yang et al.
7)8) demonstrated that artemisinin significantly inhibited the potassium outward current of
Ito channels in the Purkinje fibers by 84% at 100 μM in a concentration-dependent and reversible manner. The concentration of artemisinin used (100–150 μM) in the current study was based on that report. As
Ito channels are prominent in the RVOT epicardium, the present study examined canine wedge preparations of the RVOT, which is related to BrS.
23)
Exposure to the provocative agents caused an AP loss of dome to be observed in the epicardium. This transmural voltage gradient contributed to accentuating the J wave, appearing of ST segment elevation, and it resulted in an increase of TDR. It is important to form a phase 2 reentry circuit between the epicardium and endocardium, creating a vulnerable window. Ultimately, it leads to the development of closely coupled extrasystoles and polymorphic ventricular tachyarrhythmias. This is the ventricular arrhythmia mechanism on behalf of the repolarization hypothesis. By the suppression of potassium channels like Ito, IKr, and IKs channels, we believe artemisinin can reduce TDR that represents repolarization heterogeneity, and eventually suppress ventricular arrhythmias. After the addition of artemisinin, AP domes were recovered, ventricular arrhythmias did not reoccur, and the J wave was also diminished.
Even though artemisinin is also known to have an inhibitory effect of multiple ion channel including
INa in the previous report,
9)10) there was no conduction delay after artemisinin infusion in the present study. Thus, artemisinin does not seem to play a significant role in terms of depolarization but a suppressor role in terms of repolarization in BrS model induced by NS5806. Such findings are similar to sodium channel blocker, quinidine. We believe that among the effects on various ion channels, artemisinin might predominantly affect the outward current of potassium channels, especially
Ito channels, in the early period of AP duration.
Polymorphic ventricular tachycardias were prominently suppressed in this experiment. However, PVCs were identified in 3 of the 8 preparations, even after artemisinin. At times, some depolarization was observed only in the epicardium and, were not propagated to the endocardium. Szél and Antzelevitch
4) explained that this premature beat of the epicardium, which does not spread to other myocardium, is a concealed phase 2 reentry. In the preparations where PVCs were identified, we expect that the PVCs would have not occurred if higher concentrations of artemisinin were perfused.
The AP duration (APD) was prolonged after infusion of the provocation agents. Artemisinin showed a tendency to shorten the APD. The APD changes, AP notch index, J wave area, and TDR also showed a similar pattern to the previous study.
24) Although the exact mechanism of how
Ito channel agonists prolong APD is still unknown, the changes in APD seem to have a close relationship with
Ito channels. This presumption is supported by a study that revealed less APD prolongation was identified in tissues with light
Ito channel density.
25) NS5806, an
Ito channel agonist, usually exerts its effect on phase 1 of the AP, which means that NS5806 prolongs the duration of the phase 1 period, leading to an overall increase in APD. Additionally, Litovsky and Antzelevitch
26) demonstrated that APD was prolonged with acetylcholine until the AP plateau was suppressed. Verapamil also causes a further outward shift in the balance of the current.
3)
Other agents such as cilostazol, milrinone, isoproterenol, and flavonoids, such as acacetin, have also been reported to suppress ventricular arrhythmias and typical BrS ECG manifestations.
13)24)27) Acacetin is another flavonoid produced by plants such as the snow lotus, reported to inhibit
Ito,
IKur, and
IK-ACh channel activity.
28) Accordingly, Di Diego et al.
24) demonstrated that acacetin could suppress ventricular arrhythmia by the inhibition of
Ito channels in a BrS experimental model. Consequently, the serial changes before and after acacetin administration in APs and pseudo-ECGs were similar to the electrograms recorded in this present artemisinin study.
Our study revealed the artemisinin could suppress BrS phenotypes successfully. Considering adverse effects of other drugs, artemisinin has minimal adverse effects which might be acceptable and its safety profile was proven as it has been used as antimalarial drug for a long time. Therefore, we think it is meaningful as a drug re-position and there might be a possibility to use in real-world practice.
In conclusion, the present study suggests that artemisinin alleviates BrS phenotypes in a BrS wedge preparation model induced by NS5806 and might suppress the development of ventricular tachyarrhythmia by inhibition potassium channels including Ito channels.
When the wedge preparation is maintained for several hours, muscle fatigue can affect ventricular tachyarrhythmia inducibility. The induced ventricular tachyarrhythmia after the wash-out of artemisinin reduces this limitation.
We mimicked the BrS model by
Ito channel agonists and the model was intervened by
Ito channel antagonists. Therefore, it could be a limit to generalizing our study findings in BrS as they are, because ion channel defects of BrS are not found only in
Ito channels but also in sodium channels. Thus, this limitation might be solved in another BrS model by ajmaline and pinacidil.
29) We did not verify the
Ito current suppression by artemisinin in isolated RV epicardial myocytes by ourselves. Artemisinin inhibits not only
Ito but also
IKr and
IKs.
IKr and
IKs also may contribute to restoration of the AP dome. Thus, it is not easy to say conclusively that the antiarrhythmic effect is purely a result from
Ito suppression.
All of our findings were derived from an experimental animal model. Therefore, great caution should be taken regarding the extrapolation of the results to the clinical setting. Further in-vivo animal or clinical studies are needed to validate the possibility of using artemisinin to treat BrS. We are planning to evaluate ventricular tachycardia inducibility in artemisinin-feeding animals.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download