This article has been
cited by other articles in ScienceCentral.
Abstract
There are many reports of subacute thyroiditis (SAT) that occurred after the coronavirus disease 2019 (COVID-19), but no such case has been reported in Korea. Moreover, the simultaneous occurrence of SAT and Graves’ disease (GD) is rare. Here, we describe a patient who developed SAT and GD after the second episode of COVID-19. A 27-year-old woman with no known history of thyroid disease presented with fever, upper respiratory tract symptoms, and painful neck swelling. Thyroid function tests revealed thyrotoxicosis, and thyroid ultrasound showed heterogeneous echogenicity of enlarged thyroid glands. Her initial clinical presentation was consistent with SAT after viral infection, with typical neck tenderness and spontaneous improvement of thyrotoxicosis without antithyroid drug use. However, this case had some atypical features, such as an elevated thyroid-stimulating immunoglobulin level, relapse of thyrotoxicosis in short-term follow-up, and increased Tc-99m pertechnetate uptake, suggesting the coexistence of GD. About two months after methimazole (15 mg/day) was prescribed, she was lost to follow up again. We report the first case of unusual co-occurrence of SAT and GD following COVID-19.
Go to :

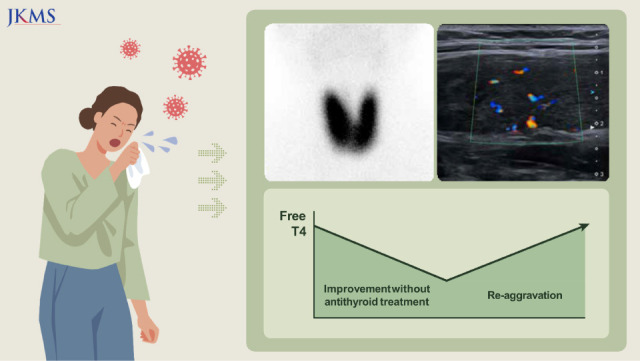
Graphical Abstract
Go to :

Keywords: Thyroiditis, COVID-19, SARS-CoV-2
INTRODUCTION
Subacute thyroiditis (SAT) is frequently preceded by a viral infection and is thought to be caused by the viral infection itself or post-infectious inflammation. Several viruses, such as the influenza virus and the Epstein-Barr virus, were reported to be associated with this self-limiting illness.
1 Several thyroiditis cases temporarily associated with coronavirus disease 2019 (COVID-19) have been reported abroad.
2345678910111213 In Korea, while a few cases of SAT after receiving a COVID-19 mRNA vaccine were reported, there has been no report of SAT following COVID-19.
1415 Although Graves’ disease (GD) is the most common cause of thyrotoxicosis in general, accounting for 60–80% of all thyrotoxicosis cases, thyrotoxicosis related to COVID-19 is mainly due to SAT, with only two cases of GD after COVID-19 reported.
16 Moreover, the simultaneous occurrence of SAT and GD is a rare phenomenon and has not been reported in association with COVID-19 in the literature yet. Here, we report a case of coexisting SAT and GD that occurred after a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Go to :

CASE DESCRIPTION
On September 17, 2022, a previously healthy 27-year-old woman with no history of thyroid disease visited our emergency department (ED) complaining of fever, sore throat, and frontal headache. She tested positive for SARS-CoV-2 twice, first in late February 2022 and then on September 4, 2022. Before the first episode of COVID-19, she had received three doses of the COVID-19 vaccine. She was afebrile but had a headache and sore throat during the second episode of COVID-19, which persisted after the end of isolation and worsened on the date of the visit.
The initial vital signs were as follows: blood pressure, 130/80 mmHg; pulse rate, 106 beats per minute; respiratory rate, 20 breaths/min; and body temperature, 37.2°C. Physical examination revealed no pharyngeal injection, tonsillar hypertrophy, or neck stiffness. The white blood cell count was 15,300 per microliter, 66.1% of which were neutrophils, and the C-reactive protein level was 31.13 mg/L. Plain radiographs of the soft tissue of the neck and chest were normal. The electrocardiogram was unremarkable except for a prolonged QT interval (QTc, 482 ms). The patient was discharged with amoxicillin/clavulanate and pelubiprofen the following day.
However, she returned to the ED on the same day, complaining of persistent symptoms despite medication. She also complained of pricking chest pain, which worsened during expiration. The blood pressure was 113/78 mmHg, pulse 142 beats per minute, respiratory rate 20 breaths per minute, and body temperature 39.3°C. An influenza antigen test yielded negative results. The creatine kinase-MB level was 0.53 ng/mL (reference range: 0–3.61 ng/mL) and troponin T was 0.004 ng/mL (reference range: 0–0.1 ng/mL). The thyroid function test (TFT) was ordered because of unexplained fever and tachycardia in an otherwise healthy young woman. Free T4 level was 3.02 ng/dL (reference range: 0.61–1.20 ng/dL) and thyroid-stimulating hormone (TSH) level was less than 0.003 uIU/mL (reference range: 0.38–5.33 uIU/mL), consistent with thyrotoxicosis. Computed tomography (CT) of the neck revealed mild enhancement and swelling of the bilateral palatine tonsils, bilateral cervical lymph node enhancement, and mild heterogeneous enhancement of both thyroid glands. The chest CT scan was normal.
The patient visited the outpatient clinic on September 19, 2022. Physical examination revealed tender swelling of the bilateral anterior neck and an enlarged left tonsil with white exudate. Due to lingering upper respiratory tract symptoms, the COVID-19 reverse transcriptase polymerase chain reaction test was ordered and returned negative. Autoantibody tests were performed for the evaluation of thyrotoxicosis. The antithyroid peroxidase (TPO) antibody titer was >1000 IU/mL, and the anti-thyroglobulin (TG) antibody was not detected. A Tc-99m pertechnetate thyroid scan revealed an enlarged bilateral thyroid with increased Tc-99m uptake (20-min Tc-99m uptake: 8.85 [reference range: 1.7–4%]) (
Fig. 1).
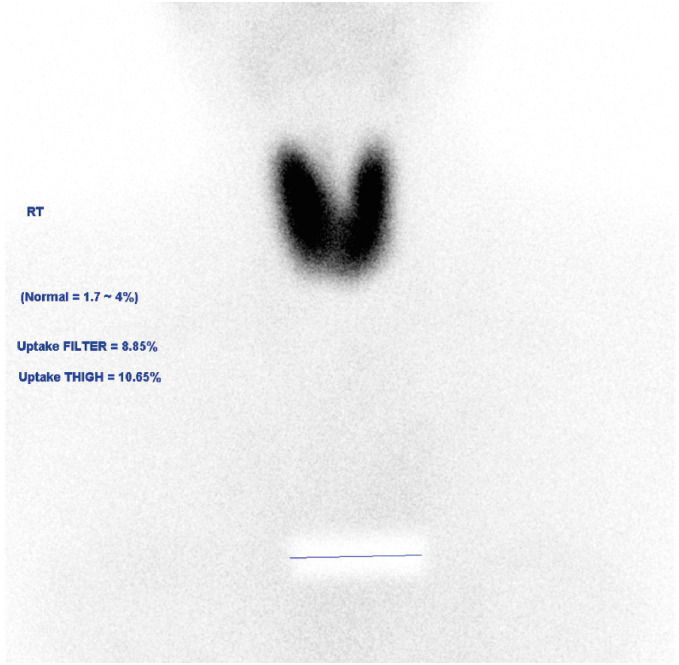 | Fig. 1 Tc-99m pertechnetate thyroid scan image.
|
When she returned to the clinic 2 days later, the tender swelling of the anterior neck, fever, and palpitation improved. She was prescribed naproxen 500 mg three times a day and propranolol 20 mg twice a day for 7 days. Ultrasonography of the thyroid gland showed architectural distortion and increased blood flow in the bilateral thyroid glands, consistent with thyroiditis (
Fig. 2). After a week of medication, the patient became afebrile, and the tender neck swelling further improved. On repeat ultrasonography performed on October 18, 2022, diffuse heterogeneous echogenicity of the bilateral thyroid glands with increased vascularity was still noted. TFT performed on the same day revealed normalization of the free T4 level (1.57 ng/dL), yet TSH was still fully suppressed (0.02 uIU/mL). An extended 28-day course of naproxen and propranolol was administered. When she returned to the clinic on October 27, 2022, the tender swelling had almost disappeared, while palpitations and tachycardia persisted. Because the increased Tc-99m uptake was not consistent with SAT, a thyroid-stimulating immunoglobulin (TSI) measurement was ordered to rule out concurrent Graves’ disease on November 29, 2022, a month later than planned because the patient did not show up for the scheduled visit. The TSI was higher than 40 IU/L (95.6%), and TFT worsened to overt thyrotoxicosis, strongly suggesting GD (
Table 1). She was referred to an endocrinologist, who prescribed methimazole (15 mg) once daily and propranolol (10 mg) twice daily. She was lost to follow up again about two months later.
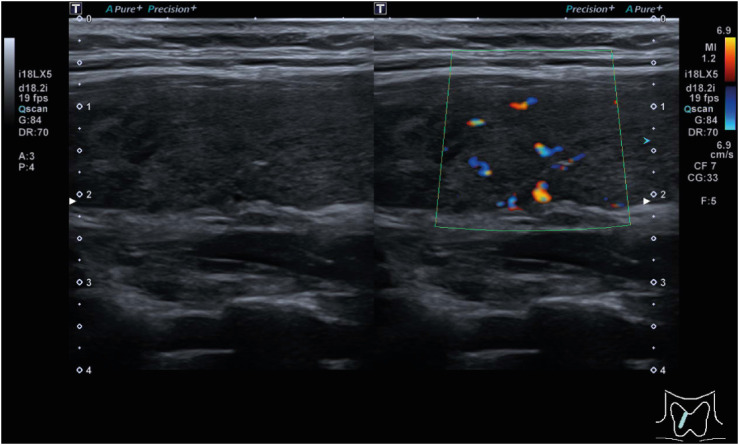 | Fig. 2 Thyroid ultrasonography image.
|
Table 1
Thyroid function tests, antithyroid antibodies, and inflammatory markers during illness
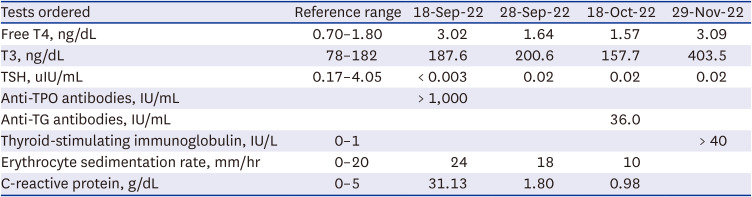
|
Tests ordered |
Reference range |
18-Sep-22 |
28-Sep-22 |
18-Oct-22 |
29-Nov-22 |
|
Free T4, ng/dL |
0.70–1.80 |
3.02 |
1.64 |
1.57 |
3.09 |
|
T3, ng/dL |
78–182 |
187.6 |
200.6 |
157.7 |
403.5 |
|
TSH, uIU/mL |
0.17–4.05 |
< 0.003 |
0.02 |
0.02 |
0.02 |
|
Anti-TPO antibodies, IU/mL |
|
> 1,000 |
|
|
|
|
Anti-TG antibodies, IU/mL |
|
|
|
36.0 |
|
|
Thyroid-stimulating immunoglobulin, IU/L |
0–1 |
|
|
|
> 40 |
|
Erythrocyte sedimentation rate, mm/hr |
0–20 |
24 |
18 |
10 |
|
|
C-reactive protein, g/dL |
0–5 |
31.13 |
1.80 |
0.98 |
|

Ethics statement
The requirement for informed consent was waived for this case report by the Institutional Review Board (IRB) of Korea University Guro Hospital (IRB No. 2022GR0421).
Go to :

DISCUSSION
Herein, we describe a case of concurrent SAT and GD that developed after COVID-19. Pain in the anterior neck with upper respiratory tract symptoms and improvement in TFT without an antithyroid drug strongly support the diagnosis of SAT. However, this case was atypical in terms of the following: 1) increased Tc-99m uptake, 2) increased vascularity on ultrasound, and 3) fluctuation of TFTs—from overt thyrotoxicosis to a subclinical state and again to overt thyrotoxicosis in the short term of 2 months. Considering this, the possibility of concomitant GD cannot be excluded. After a brief improvement without antithyroid treatment, the thyrotoxicosis recurred. The diagnosis of GD was confirmed by a high TSI.
To the best of our knowledge, there is only one case in the literature in which SAT and GD occurred simultaneously.
17 Like our case, this patient presented with neck pain, elevated anti-TPO antibody level, a thyroid scan revealed areas of increased uptake, thyroid ultrasound revealing increased vascularity, and symptoms that improved without an antithyroid drug. Although TSH receptor antibody levels were markedly elevated in this patient, fine-needle aspiration biopsies revealed granulomatous thyroiditis, which is the hallmark of SAT. The authors suggested genetic susceptibility and autoimmunity triggered by SAT itself or a preceding viral infection as possible mechanisms.
Several autoimmune thyroid diseases after SARS-CoV-2 infection have been reported since the early phase of the COVID-19 pandemic. SARS-CoV-2 uses angiotensin-converting enzyme 2 combined with the transmembrane protease serine 2 as the key molecular complex to infect the host cell, which is highly expressed in both the thyroid gland and the lung.
18 In addition to the many cases of SAT, newly developed Hashimoto’s thyroiditis and GD have also been reported.
161920 These cases largely followed the typical disease courses of each diagnosis. To date, most cases of post-COVID SAT have features similar to those of non-COVID-related cases. Most show a typical clinical course, negative autoantibodies, reduced vascularity, and reduced radionuclide uptake. However, some researchers have suggested possible differences between post-COVID thyroiditis and pre-pandemic thyroiditis, in that there might be an overlap between post-COVID thyroiditis and autoimmune thyroid disease.
89 Feghali et al.
9 reported a case of post-COVID hypothyroidism, which was atypical of Hashimoto’s thyroiditis in that a fine-needle biopsy revealed granulomatous inflammation without clear lymphocytic infiltration. Despite high titers of anti-TPO and anti-TG antibodies. It cannot be excluded that our patient might have experienced such a rare simultaneous occurrence of the two conditions due to COVID-19.
A notable limitation of our study is that TSI was not measured when thyrotoxicosis was first diagnosed. If TSI elevation was confirmed at that time, it would have been a clearer evidence of simultaneous occurrence of SAT and GD. Therefore, when a patient who recently contracted COVID-19 presents with thyrotoxicosis, physicians should thoroughly evaluate the possibility of both SAT and GD. A recent history of viral illness and the presence of anterior neck pain and tenderness should not rule out GD.
In conclusion, we reported a case of concurrent SAT and GD after SARS-CoV-2 infection. It would be helpful for clinicians to know that thyroid diseases, including thyroiditis, can occur after COVID-19 and that this may be associated with autoimmunity, either preexisting or triggered by the virus. Further studies on the possible association between COVID-19 and autoimmune thyroid disease are warranted.
Go to :