A 48-year-old male without any underlying diseases was referred to our department of periodontology due to an abnormality in the right buccal gingiva after a scaling procedure at a local clinic. Since no improvement in symptoms occurred after periodontal treatment, the patient was referred to the department of oral and maxillofacial surgery. A panoramic radiograph revealed alveolar bone resorption of #43 to #45.(
Fig. 1) A biopsy was performed, and diagnosis of spindle cell tumors, including inflammatory myofibroblastic tumors (IMT), was based on immunoreactivity for ALK. Magnetic resonance imaging (MRI) and CECT (contrast-enhanced computed tomography) were performed but revealed no abnormal findings. Due to the swelling, redness, and pain in the affected area without improvement in symptoms, marginal mandibulectomy was performed on the patient under general anesthesia.
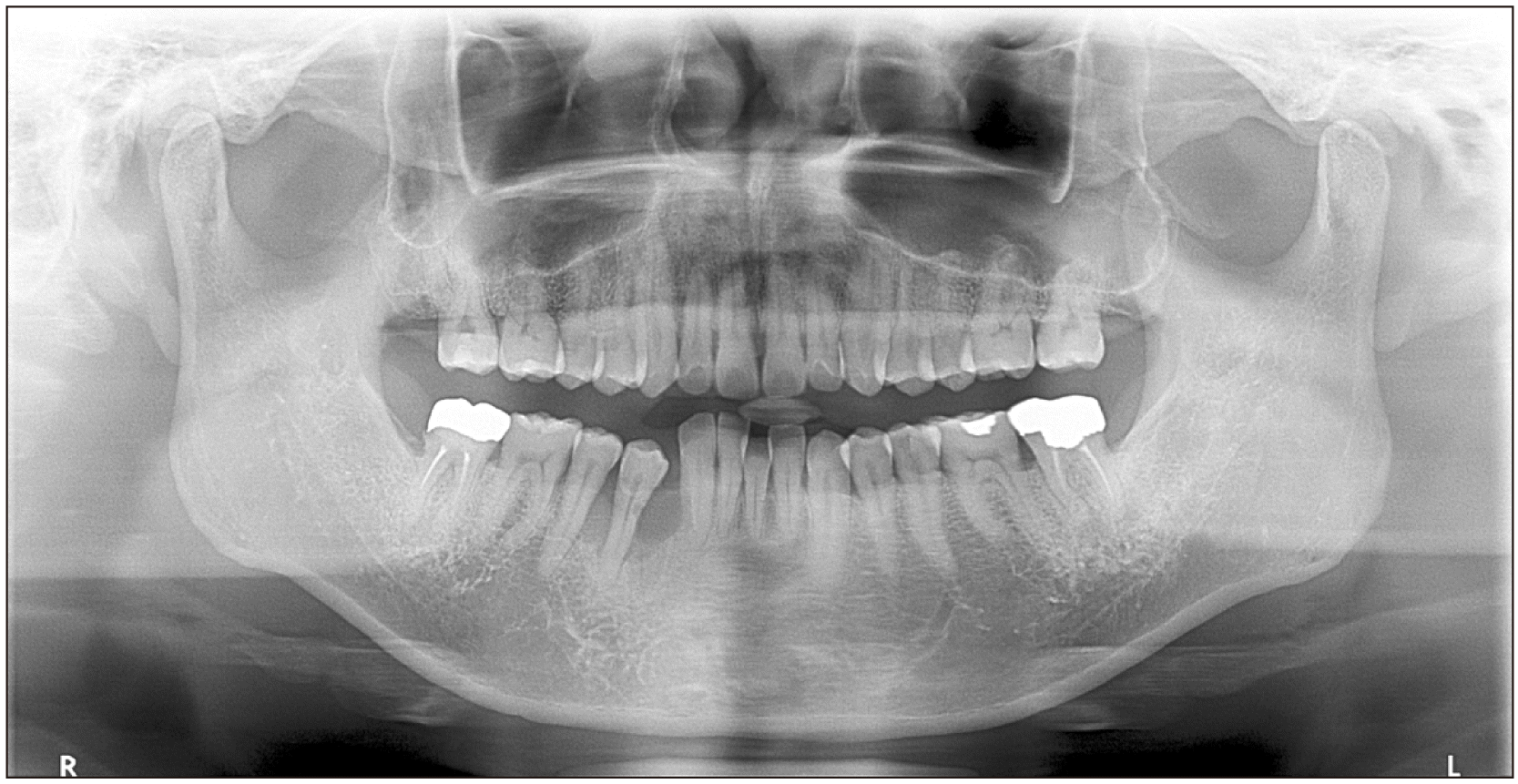 | Fig. 1Preoperative panoramic radiograph. 
|
The pathologic findings were the same in all biopsied and resected specimens. All microscopic slides were reviewed with additional immunohistochemical staining and a thorough literature review. Histological examination revealed fascicular proliferation of spindled tumor cells at the subepithelial portion of the gingiva.(
Fig. 2. A) Tumor cells contained eosinophilic cytoplasm and possessed blunted, ovoid, or fusiform nuclei with inconspicuous nucleoli and occasional mitotic figures.(
Fig. 2. B) Sclerosing or focal myxoid stroma was noted. The overlying gingival mucosa exhibited erosion accompanied by mixed inflammatory infiltrate with abundant plasma cells. The inflammatory infiltrate was prominent below the erosive mucosa (
Fig. 2. C) but was inconspicuous in the deep portion of the tumor. The marginal mandibulectomy specimen revealed that the tumor cells had invaded the mandibular bone trabeculae. On immunohistochemistry, the tumor cells were strongly and diffusely positive for vimentin, desmin (
Fig. 2. D), and myoD1 (
Fig. 2. E) and were strongly and multifocally positive for smooth muscle actin (SMA), caldesmon, ALK (
Fig. 2. F), and cytokeratin. Tumor cells were negative for epithelial membrane antigen (EMA), myoglobin, S100 protein, and p53. The Ki67-labeling index was approximately 20%. Based on the combined histological and immunohistochemical findings, a revised diagnosis of SCRMS with ALK expression was concluded. No recurrent lesions were observed for 8 months after surgery. Postoperative PET/CT (positron emission tomography/computed tomography) and MRI revealed no residual tumors and no distant or lymph node metastases.
 | Fig. 2A. Photomicrograph reveals proliferation of fascicular spindled tumor cells in the subepithelial portion (H&E staining, ×100). B. Higher magnification of tumor cells showing blunted, elongated nuclei and abundant eosinophilic cytoplasm with inconspicuous inflammation (H&E staining, ×200). C. Abundant inflammatory infiltrate associated with mucosal erosion (H&E staining, ×200). D. Tumor cells diffusely positive for desmin (immunohistochemistry staining, ×200). E. Tumor cells diffusely positive for myoD1 (immunohistochemistry staining, ×200). F. Tumor cells multifocally positive for anaplastic lymphoma kinase (immunohistochemistry staining, ×200). 
|





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download