I. Introduction
Osteoporosis is a skeletal disease characterized by low bone mass and disruption of bone architecture that compromise bone strength and increase fracture risk. When treating this disease, antiresorptive agents have been used as the first-line therapy, one of which is bisphosphonates (BPs)
1. However, long-term use of BPs has been associated with osteonecrosis of the jaw (ONJ) in a condition known as medication-related osteonecrosis of the jaw (MRONJ)
2.
The pathomechanism of MRONJ is multifactorial and not fully understood, yet several potential mechanisms have been mentioned in the literature, including suppression of bone remodeling and blood flow imbalance due to excessive inhibition of osteoclast activity by BPs, direct toxicity of BPs to soft tissue, infection, and changes in immune surveillance, as well as the antiangiogenic effects of BPs
3-5. Local factors, including invasive oral surgery, thin mucosa, and periodontal disease, and systemic factors, such as use of steroids, age, diabetes, genetic factors, BP type, and use for longer than 3 years, increase the risk of MRONJ
4,6.
The suspension of BPs before surgery to reduce the risk of MRONJ is recommended in several studies
5,7,8. The idea of suspending BPs arose from two basic principles: first, prolonged use of BPs has been reported as a risk factor for MRONJ and, second, after use of BPs over a given period of time, no additional benefit is found
9. Marx proposed a 6-month suspension of BPs based on the increased collagen type I C-telopeptide level
10. Damm and Jones
11 proposed a suspension of BPs for 2 months before oral surgery based on bone physiology and pharmacokinetics because free BP level in the serum was believed to be extremely low at 2 months after the last dose of oral BP. The 2014 position paper of the American Association of Oral and Maxillofacial Surgeons (AAOMS) also considered BPs suspension of BPs for 2 months before surgery
7. In 2015, a position statement of the Korean Society for Bone and Mineral Research (KSBMR) and the Korean Association of Oral and Maxillofacial Surgeons (KAOMS) suggested BP suspension 2-4 months before surgery
8. Several clinical and meta-analysis articles have reported suspension of BPs as a good prognostic factor for MRONJ treatment outcome
12-15. In contrast, several studies did not find a benefit in suspending BPs on treatment outcomes and suggested that more studies are needed on this matter
3,16,17. Due to a deficiency in clinical evidence, the efficacy of BP suspension before surgery remains controversial. The purpose of this study was to quantitatively evaluate the clinical significance of BP suspension before surgery in improving the outcome of MRONJ treatment in patients with osteoporosis.
Go to :

II. Materials and Methods
1. Patients and study design
From January 2012 to January 2020, all patients with MRONJ treated by one surgeon at the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital were analyzed. The current study, including access to patient medical records, received ethical approval from the Institutional Review Board of Seoul National University (S-D20160039). The study was conducted in accordance with the relevant guidelines and regulations of the Declaration of Helsinki, and written informed consent was obtained from all participants. The MRONJ diagnosis was established through patient medical history, clinical evaluation, radiologic findings, and histopathological examination, and the stage of disease was determined according to the AAOMS guidelines
2,7,18.
Patients with acute pain, extraoral pus discharge, dysphagia, pathologic fracture, and suspected malignant lesions were treated as soon as possible without drug suspension, and the treatment outcomes were compared with that of patients who suspended drugs for at least three months before surgery.
1) Inclusion criteria
(1) Patients with osteoporosis who were taking or had taken BPs.
(2) Patients with complete clinical data, including periodic follow-up panoramic radiographs and laboratory results.
2) Exclusion criteria
(1) Patients who were diagnosed and previously or currently treated for osteoporosis with BPs concomitant with denosumab.
(2) Cancer patients.
(3) Patients with incomplete radiographs, and those who were lost during follow-up.
2. Treatment protocol
Upon patient arrival at the clinic, records of the systemic condition, previous dental treatment and medical history, and medication prescriptions, including BPs, were checked. Clinical and radiograph assessments were performed to evaluate the disease stage.
Treatment was administered according to the disease stage, as follows.
• Stage 0: Systemic therapy using antibiotics, pain management with analgesics, oral hygiene maintenance, and removal of risk factors, such as minimally invasive extraction of a hopeless tooth.
• Stage 1: Surgical curettage, systemic therapy using antibiotics and pain medication.
• Stage 2: Saucerization with or without reconstruction plate (R-plate) placement, systemic therapy using antibiotics and pain medication.
• Stage 3: Saucerization with or without R-plate placement, modified endoscopic sinus surgery (MESS) for cases with sinus involvement, systemic therapy using antibiotics and pain medication.
Oral antibiotic from the beta-lactam class, including cephalosporin or penicillin with clavulanate, was prescribed to the patients for at least 2 weeks before surgery and continued for at least 7 days following surgery. Primary wound closure was performed for all cases. Periodic follow-up appointments were scheduled to observe the healing progress through clinical and radiograph evaluation at 3 months, 6 months, and 1 year postoperatively. All patients were instructed to discontinue BPs following surgery for as long as they were under supervision.
3. Number of interventions
In this manuscript, the number of interventions was defined as the total surgical procedures that the patient received. We collected data regarding the number of interventions from admission until final follow-up. The obtained data were compared between groups.
4. Clinical outcome
The clinical outcome was measured by comparing preoperative and final postoperative ONJ stage (AAOMS). The results were categorized into four groups
19.
• Absent ONJ lesion: uneventful healing with intact mucosa and no signs of inflammation after single or multiple treatments.
• Reduced ONJ lesion: partially exposed bone (postoperative< preoperative stage).
• Unchanged ONJ lesion: no significant improvement (postoperative=preoperative).
• Increased ONJ lesion: progression of the disease despite surgical intervention (postoperative>preoperative).
In the absence or reduction of ONJ lesions, the treatment outcome was considered successful; in unchanged or increased ONJ lesions, the treatment was considered unsuccessful.
5. Relative bone density measurements
The relative bone density measurement was used in this study to evaluate the healing process of the jaw through easy digitalized panoramic analysis expressed as gray values
20,21. The gray values were saved as 8-bit images with values ranging from 0 to 255, where 0 indicates low radiologic density (black) and 255 indicates high density (white)
20.
An Orthopantomograph OP100 (Instrumentarium Corp.) was used to obtain the panoramic radiographs. We collected postoperative panoramic radiographs immediately, three months, six months, and one year after the first procedure and analyzed them for bone density using a scientific image-analysis program (Fiji ImageJ software, version 1.53c; NIH).
To obtain the gray values, a rectangle annotation was used for the region of interest (ROI). The ROI was set at the center of the defect area in the jaw using a 50 mm
2 rectangular selection tool. The contralateral bone on the unaffected side was used reference values
21.(
Fig. 1) Foreign bodies, such as titanium plates, implants, and drains, as well as other anatomic structures, including teeth, mandibular canal, mental foramen, and maxillary sinus, were not included in the selection area. This step was repeated three times, and the mean gray value (MGV) was calculated. The relative bone density value was calculated as the quotient of the MGV of the ROI and of the contralateral side
20.
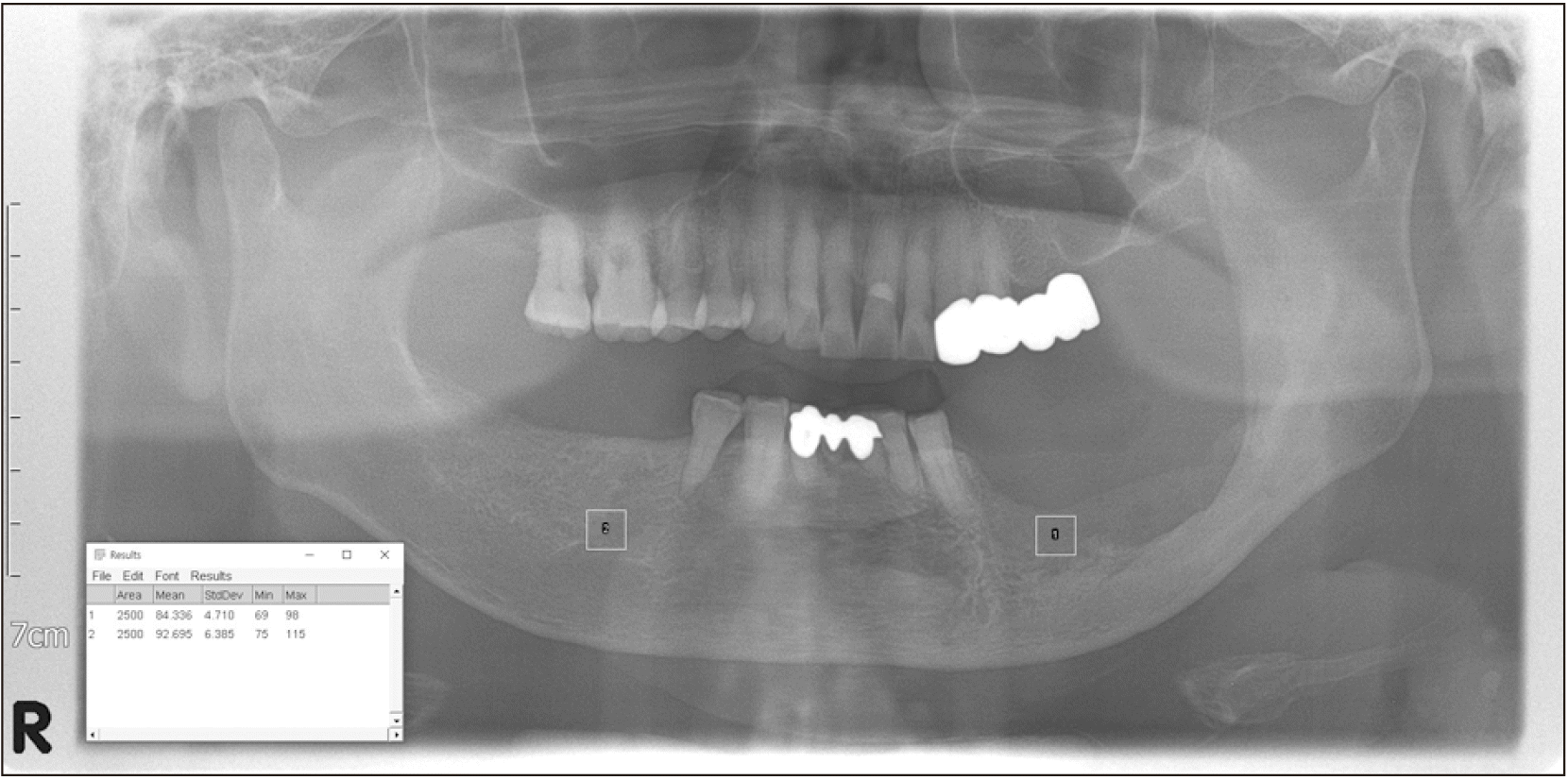 | Fig. 1The method used in the study to quantify relative bone density using Fiji ImageJ software (ver1.53c; NIH). 
|
6. Laboratory analysis
We collected laboratory data from the medical records of the patients including white blood cells (WBC), erythrocyte sedimentation rate (ESR), absolute neutrophil count (ANC), hemoglobin (Hb), hematocrit (Hct), and alkaline phosphatase (ALP), preoperatively and postoperatively. The preoperative laboratory results were collected on the first visit of the patient to the clinic. The postoperative laboratory results were obtained 1-3 days after surgery. In our institution, the normal ranges of WBC, ESR, ANC, Hb, Hct, and ALP are 4-10×103/µL, 0-20 mm/h, 1,800-7,000/µL, 12-16 g/dL, 39%-52%, and 30-115 IU/L, respectively. We compared the preoperative and postoperative results and performed correlation tests between the changes in ESR and ALP.
7. Statistical considerations
The data were analyzed statistically using IBM SPSS Statistics program (ver. 26.0; IBM). For number of interventions, a Mann–Whitney U test was used for comparison between groups. Fisher’s exact test was used to measure the association between treatment outcome and medication discontinuation. For relative bone density, repeated measures of ANOVA were used in the normally distributed group, and Student’s t-test was used for comparison between groups. For laboratory results, Student’s t-test was used for comparison within and between groups. A Pearson correlation test with bootstrapping was performed for changes in ESR and ALP. Statistical significance was set at P<0.05.
Go to :

III. Results
1. Patient data
After considering the inclusion and exclusion criteria, we analyzed 24 patients with MRONJ (
Table 1), 18 of these had suspended BPs for at least 3 months before surgery, whereas 6 did not and were treated as early as possible. Of these patients, 22 were female and 2 were male, with a mean age of 76.46±4.86 years, ranging from 68 to 85 years.
Table 1
Clinicopathological features of MRONJ in this study
|
Variable |
Drug suspension (n=18) |
Non-drug suspension (n=6) |
|
Sex |
|
|
|
Male |
1 |
1 |
|
Female |
17 |
5 |
|
Age (yr) |
76.17±5.17 (68-85) |
77.33±3.64 (73-82) |
|
BP type by subclass |
|
|
|
Alendronate |
8 (44.4) |
3 (50.0) |
|
Risedronate |
4 (22.2) |
1 (16.7) |
|
Ibandronate |
9 (50.0) |
2 (33.3) |
|
Pamidronate |
- |
- |
|
Zoledronate |
1 (5.6) |
- |
|
Route of administration |
|
|
|
Oral |
10 (55.6) |
6 (100) |
|
IV |
6 (33.3) |
- |
|
Oral+IV |
2 (11.1) |
- |
|
Duration of BP use (yr) |
4.40±2.70 (0.5-10) |
8.00±6.30 (3-20) |
|
Duration of BP suspension (mo) |
5.64±3.37 (3-12) |
- |
|
Preoperative stage |
|
|
|
0 |
1 (5.6) |
- |
|
1 |
- |
- |
|
2 |
10 (55.6) |
2 (33.3) |
|
3 |
7 (38.9) |
4 (66.7) |
|
Outcome assessment |
|
|
|
Absent |
13 (72.2) |
- |
|
Reduced |
2 (11.1) |
2 (33.3) |
|
Unchanged |
3 (16.7) |
2 (33.3) |
|
Increased |
- |
2 (33.3) |
|
Trigger |
|
Spontaneous |
2 (11.1) |
- |
|
Odontogenic infection |
5 (27.8) |
- |
|
Tooth extraction |
9 (50.0) |
1 (16.7) |
|
Implant procedure |
2 (11.1) |
- |
|
Implant removal |
- |
3 (50.0) |
|
Peri-implantitis |
- |
1 (16.7) |
|
Ill-fitting denture |
- |
1 (16.7) |
|
Other systemic diseases |
|
None |
2 (11.1) |
2 (33.3) |
|
Hypertension |
10 (55.6) |
3 (50.0) |
|
Diabetes mellitus |
5 (27.8) |
3 (50.0) |
|
Cardiovascular |
1 (5.6) |
- |
|
Hyperlipidemia |
1 (5.6) |
1 (16.7) |
|
Rheumatoid arthritis |
1 (5.6) |
- |
|
Pulmonary disease |
1 (5.6) |
- |
|
Smoking |
- |
- |

Regarding location, the right posterior mandible was most frequently affected (37.5%), followed by the left posterior mandible (20.8%), anterior mandible (12.5%), right posterior maxilla (12.5%), left posterior maxilla (8.3%), anterior maxilla (4.2%), and midpalate (4.2%). Regarding the stage of disease, 55.6% of the patients in the suspension group were diagnosed with stage 2 MRONJ (n=10), whereas 66.7% of the patients in the non-drug suspension group were diagnosed with stage 3 MRONJ (n=4).
2. Systemic condition
Regarding comorbidity and co-medication, excluding osteoporosis, the majority of patients was diagnosed with and treated for 1 to 6 systemic diseases. Hypertension (n=13; 54.2%) and diabetes mellitus (n=8; 33.3%) were predominant in both groups.
3. BPs medication profile
In this study, 12.5% of patients were medicated with 2 or 3 types of BP (n=3), with alendronate (n=11) and ibandronate (n=11) the most common, followed by risedronate (n=5) and zoledronate (n=1). Based on the route of administration, all patients in the non-suspension group received BPs orally (n=6). In the suspension group, 55.6% of the patients received BPs orally (n=10), 33.3% received IV BPs (n=6), and 11.1% of the patients received both oral and IV BPs (n=2). Based on the dose, 70 mg/week of alendronate, 150 mg/month of oral ibandronate, 3 mg/3 mL/3 months of IV ibandronate, 35 mg/week and 150 mg/month of risedronate, and 5 mg/100 mL/year of zoledronate were recorded in the medical records of patients.
In the suspension group, the mean medication duration was 4.40±2.70 years, ranging from 6 months to 10 years, with the duration of suspension ranging from 3 to 12 months (mean duration, 5.64±3.37 months). Meanwhile, in the non-suspension group, the mean medication duration was 8.00±6.30 years, ranging from 3 to 20 years.
4. Local factors potentially triggering the onset of MRONJ
Local factors potentially triggering the pathogenesis of MRONJ in this study were tooth extraction (n=10), odontogenic infection (n=5), implant removal (n=3), implant procedure (n=2), periimplantitis (n=1), and ill-fitting dentures (n=1). The spontaneous onset of MRONJ was found in 2 patients from the suspension group. In the suspension group, 50.0% of cases were triggered by tooth extraction (n=9), whereas in the non-drug suspension group, 50.0% of cases were triggered by implant removal (n=3).
5. Number of interventions
In total, there were 62 interventions. The highest number of interventions was 14 in 1 patient of the non-drug suspension group. The lowest number of interventions was observed in the BP suspension group, with only 1 intervention each in 14 patients.(
Table 2) Saucerization was the most used surgical approach, in 87.5% of cases (n=21). MESS with saucerization was performed in 8.3% of cases (n=2), and tooth extraction was performed in one patient with stage 0 (4.2%). In total, 9 patients receive an R-plate, 27.8% cases in the drug suspension group (n=5) and 66.7% cases in the non-drug suspension group (n=4). A significant difference in the number of interventions was observed between the suspension and non-suspension groups (
P<0.05).(
Table 3)
Table 2
Number of interventions in patients
|
No. |
Sex/age (yr) |
Initial stage |
Discontinuation duration (mo) |
No. of interventions |
Treatment outcome assessment |
|
1 |
F/74 |
2 |
3 |
1 |
Unchanged |
|
2 |
F/82 |
2 |
4 |
1 |
Absent |
|
3 |
F/83 |
2 |
6 |
1 |
Absent |
|
4 |
F/83 |
2 |
12 |
1 |
Absent |
|
5 |
F/74 |
2 |
4.5 |
1 |
Absent |
|
6 |
F/83 |
2 |
4 |
1 |
Absent |
|
7 |
F/74 |
2 |
12 |
1 |
Absent |
|
8 |
F/78 |
3 |
4 |
1 |
Absent |
|
9 |
F/74 |
3 |
3 |
1 |
Absent |
|
10 |
F/72 |
3 |
3 |
1 |
Absent |
|
11 |
F/79 |
2 |
11 |
1 |
Absent |
|
12 |
F/85 |
2 |
3 |
1 |
Absent |
|
13 |
F/68 |
2 |
5 |
1 |
Absent |
|
14 |
F/74 |
3 |
4 |
3 |
Unchanged |
|
15 |
F/76 |
3 |
5 |
2 |
Reduced |
|
16 |
F/68 |
3 |
12 |
2 |
Unchanged |
|
17 |
F/70 |
3 |
3 |
3 |
Reduced |
|
18 |
M/74 |
0 |
3 |
1 |
Absent |
|
19 |
F/78 |
3 |
- |
8 |
Unchanged |
|
20 |
F/75 |
2 |
- |
14 |
Increased |
|
21 |
F/82 |
3 |
- |
2 |
Reduced |
|
22 |
M/74 |
3 |
- |
2 |
Unchanged |
|
23 |
F/73 |
3 |
- |
11 |
Increased |
|
24 |
F/82 |
2 |
- |
1 |
Reduced |

Table 3
Comparison of the number of interventions between suspension and no suspension of BPs
|
Mean±SD |
P-value |
|
Suspension of BPs |
1.33±0.69 |
0.003*,1
|
|
No suspension of BPs |
5.71±5.25 |
|

6. Clinical outcomes
The success rate in the drug suspension group was 83.3% (n=15), compared to 33.3% in the non-drug suspension group (n=2). Unchanged lesions of MRONJ were found in 16.7% of patients in the drug suspension group (n=3) and 33.3% of patients in the non-drug suspension group (n=2). The severity of MRONJ increased in 33.3% of patients in the non-drug suspension group (n=2). Based on Fisher’s exact test, the P-value was 0.038 (P<0.05), indicating a significant association between a successful outcome and suspension of BP medication.
7. Relative bone density results
We analyzed the changes in relative bone density in the patients immediately postoperatively (T0) and 3 months (T1), 6 months (T2), and 1 year (T3) following the initial surgery. The relative bone density in the drug suspension group showed significant difference over time (
P<0.05), with one-year follow-up showing the highest relative bone density. On the other hand, in the non-drug suspension group, no significant differences were observed.(
Table 4) Significant differences were observed in T2-T0 (Δ2) and T3-T0 (Δ3) between the drug suspension and non-drug suspension groups (
P<0.05).(
Table 4,
Fig. 2,
3)
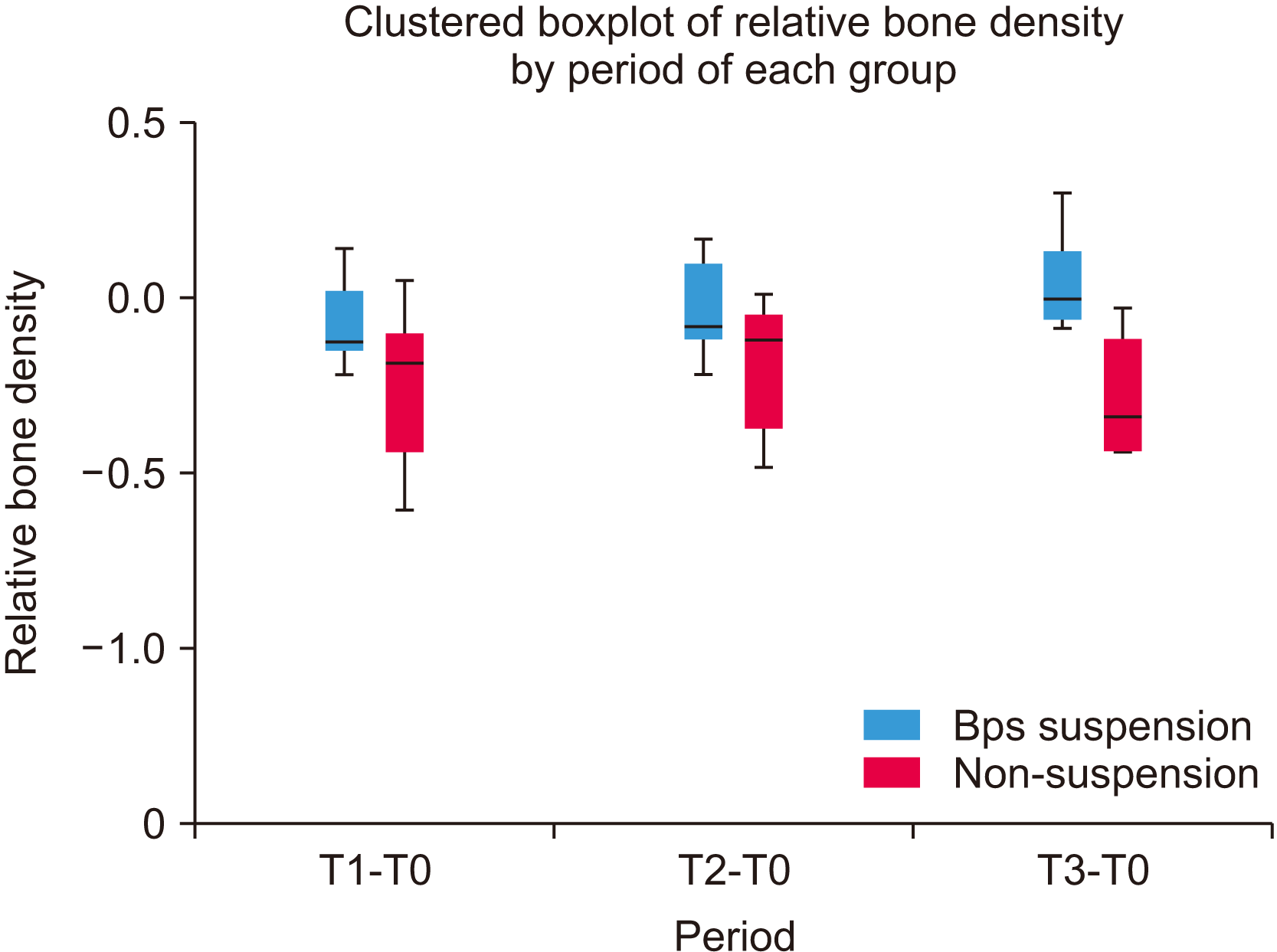 | Fig. 2The chart shows the comparison of relative bone density changes between groups in the examined time periods. The box plots show high relative bone density in the drug suspension group for every time period, indicating bone healing. On the contrary, recurrence and more frequent surgical interventions were predominant in the non-drug suspension group. In this case, the box plots of the non-drug suspension group show various levels of relative bone density. When comparing the two groups at every time period, significant differences in relative bone density levels can be observed mainly in T2-T0 and T3-T0 (P<0.05). (BP: bisphosphonate, T0: immediately postoperatively, T1: 3-month follow-up, T2: 6-month follow-up, T3: 1-year follow-up) 
|
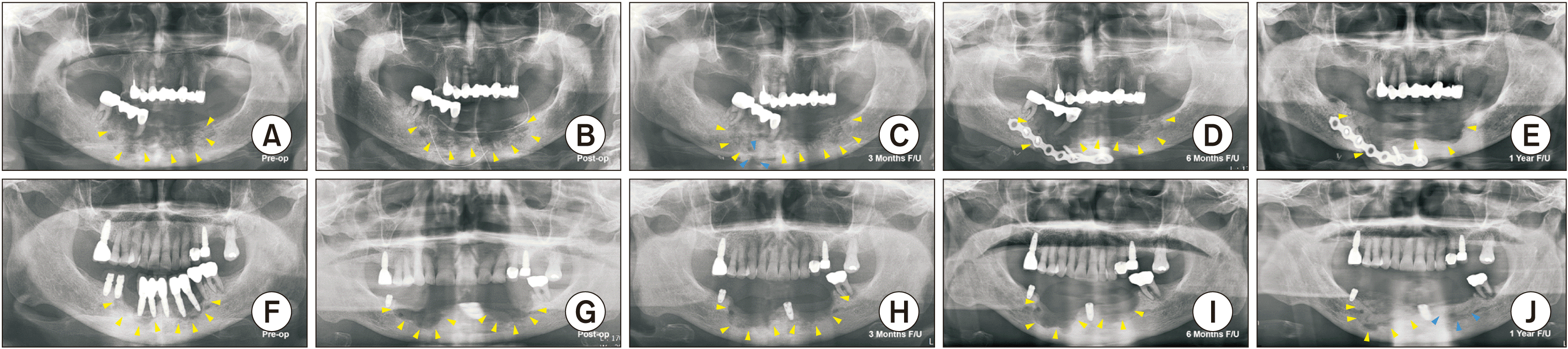 | Fig. 3Representative cases of medication-related osteonecrosis of the jaws (MRONJ) from the two groups, non-suspension (A-E) and BP suspension (F-J), showing the bone healing progress of two female patients who received oral alendronate 70 mg/week and developed stage 3 MRONJ on the anterior mandible. In the preoperative panoramic radiographs of both patients (A, F), the yellow arrowheads indicate lesion extension. The immediate postoperative panoramic radiographs of both patients (B, G). The three-month follow-up panoramic radiograph revealed signs of pathologic fracture at the base of the mandible in patients who did not suspend BPs (C, blue arrowheads) compared to patients who suspended BPs three months before surgery (H). The six-month follow-up panoramic radiographs revealed extensive expansion of the recurred lesion in patients who did not suspend BPs (D) compared to the patients who suspended BPs, which showed the bone healing process (I). The one-year follow-up panoramic radiograph of a patient who did not suspend BPs (E) and a patient who suspended BPs revealed good and almost complete bone healing on the left anterior mandible (blue arrowheads) (J). 
|
Table 4
Comparison of relative bone density within groups over periodic follow-up and relative bone density changes postoperatively between groups
|
Suspension of BPs (MGV) |
No suspension of BPs (MGV) |
P-value |
|
MGV over periodic F/U |
|
|
|
Postoperative (T0) |
0.87±0.18 |
1.04±0.15 |
|
|
3-month F/U (T1) |
0.93±0.15 |
0.97±0.14 |
|
|
6-month F/U (T2) |
0.97±0.16 |
1.01±0.12 |
|
|
1-year F/U (T3) |
1.02±0.16 |
1.03±0.12 |
|
|
P-value |
0.000*,1 |
0.287 |
|
|
MGV changes from postoperative |
|
|
|
Δ1 (T1-T0) |
0.07±0.09 |
–0.07±0.18 |
0.098 |
|
Δ2 (T2-T0) |
0.11±0.10 |
–0.03±0.15 |
0.036*,2
|
|
Δ3 (T3-T0) |
0.15±0.10 |
–0.20±0.36 |
0.003*,2
|

8. Laboratory results
We analyzed preoperative and postoperative inflammatory markers (WBC, ESR, ANC, Hb, and Hct) and ALP. There was no significant difference observed in WBC, ANC, Hb, and Hct. The postoperative ESR value in the non-drug suspension group increased non-significantly, whereas, in the drug suspension group, postoperative ESR significantly decreased (
P<0.05). Regarding the changes in ALP, although they were within the normal range, the postoperative ALP in the non-drug suspension group showed no significant increase postoperatively (
P<0.05). Meanwhile, ALP in the drug suspension group showed a significant decrease postoperatively (
P<0.05).(
Table 5) The changes in ALP occurred in tandem with the changes in ESR, and Pearson’s correlation test was performed with bootstrapping. In this study, there was a moderate positive correlation (
r=0.474,
P<0.05) between the changes of ESR and ALP preoperatively and postoperatively in all patients. This correlation was further emphasized through the positive values of the confidence interval.(
Table 5,
Fig. 4)
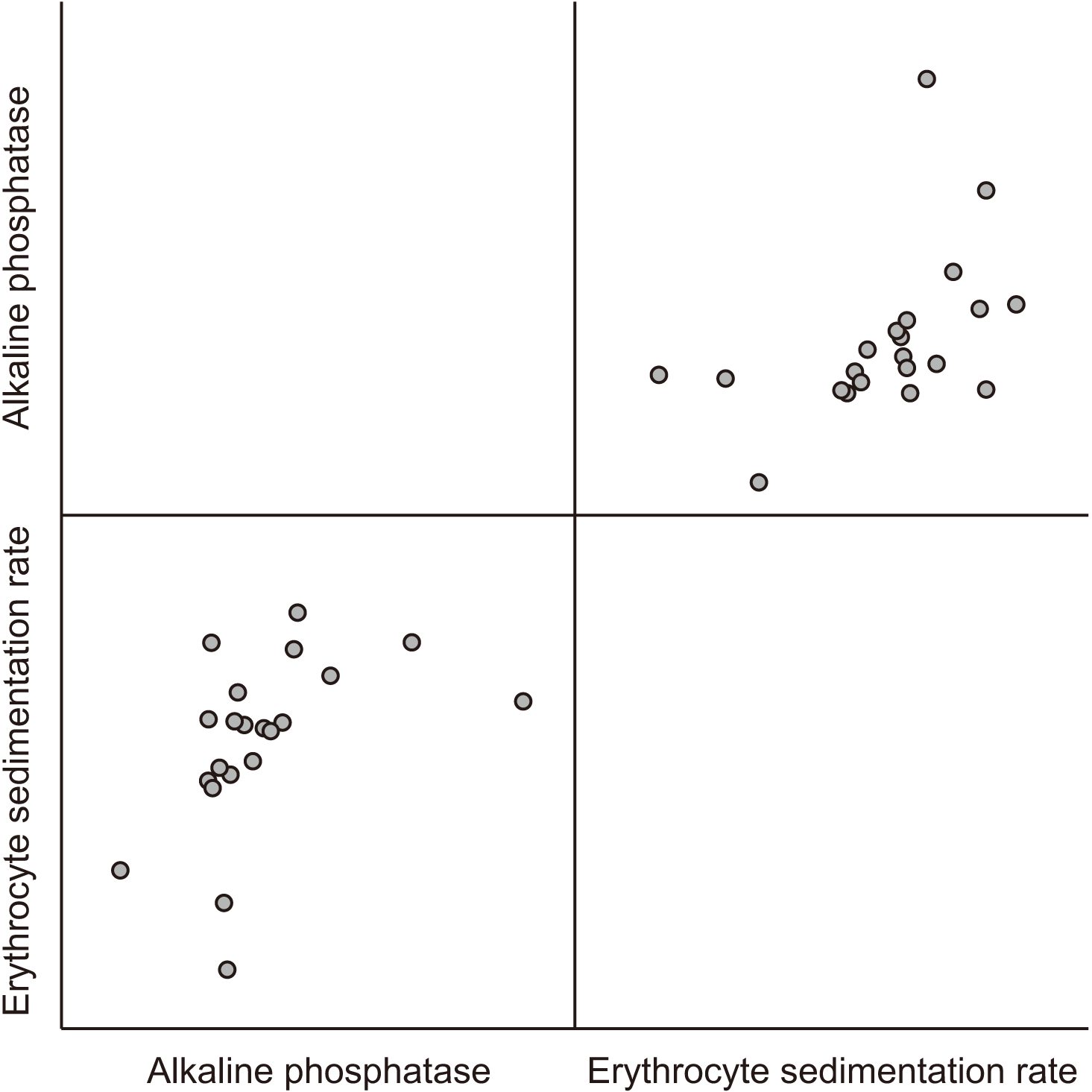 | Fig. 4Scatterplot matrix that shows a moderate degree of positive correlation (r=0.474, P<0.05) between the changes of preoperative and postoperative erythrocyte sedimentation rate (ESR) and alkaline phosphatase (ALP) in all patients. The changes in ALP were occurred in tandem with the changes in ESR. 
|
Table 5
Preoperative (Pre) and postoperative (Post) ESR and ALP results in patients with MRONJ and correlation test between changes of ESR and ALP among patients
|
ESR |
|
ALP |
|
|
|
Pre |
Post |
Pre |
Post |
|
Suspension of BPs |
47.60±31.39 |
27.80±22.971 |
70.80±17.09 |
59.40±10.481 |
|
No suspension of BPs |
38.57±26.03 |
40.57±30.62 |
60.29±11.12 |
77.57±23.01 |
|
r
|
P-value |
95% CI |
|
ΔESR |
0.474 |
0.026*,2
|
0.251 |
0.776 |
|
ΔALP |
|
|
|
|

Go to :

IV. Discussion
Since 2004, MRONJ in osteoporosis patients has been associated with the use of BPs
22. Bone metabolism is mainly regulated by sex steroid hormones, especially estrogen, which has a direct effect on osteocytes, osteoblasts, and osteoclasts
23. For this reason, the incidence of osteoporosis is higher in postmenopausal women than in men. However, the incidence of osteoporosis in men should not be overlooked, as it can arise due to and may manifest as a secondary disease
24. Based on this principle, MRONJ in osteoporosis patients was mostly found in women. Our study revealed a high incidence of MRONJ in postmenopausal women, accounting for 91.7% of all cases.
MRONJ is only found in the mandible and maxilla, which highlights the uniqueness of the jawbones compared to peripheral bones. The bone turnover of jaws is 10-fold greater than in other skeletal bones
25. In addition to different modes of ossification between jawbones and peripheral bones, jawbones are near the open environment of the oral cavity and bear increased stress from mastication
26. Antiresorptive agents increase bone mass but cause extensive ossification and trabecular connectivity loss to the jawbones, leading to microcracks
27. The mandible is a stress-bearing structure that experiences load and microdamage
26. As opposed to the mandible, the maxilla has a more complex vascular network and abundant blood supply that fosters healing and is less affected by MRONJ
13. For these reasons, and in line with the literature
13,28, the mandible is the site most affected by MRONJ, accounting for 70.8% of the present cases, 2.43 times more frequent than in the maxilla. Furthermore, MRONJ in the posterior part of the mandible is more common than in the anterior part
5.
According to previous studies, the occurrence of MRONJ after receiving IV BPs was 0.8% to 12%, whereas that for oral BPs was 0.00104% to 0.00169%
13,14. Although the percentage of MRONJ in patients taking oral BPs is small, most patients in the study had stage 2 or 3 MRONJ, especially those in the non-suspension group, who received BPs through an oral route rather than through IV. Therefore, the potential of oral BPs causing MRONJ should not be overlooked. According to the database of the Korean National Health Insurance, alendronate was the most frequently prescribed medication for osteoporosis, followed by risedronate, ibandronate, pamidronate, and zoledronate
29. In another study, ibandronate was the most common BP for treating osteoporosis
6. We found alendronate and ibandronate to be the most used antiresorptive agents. Risedronate had the highest potency, followed by ibandronate and alendronate. Based on bone-binding affinity, alendronate has the strongest affinity, followed by ibandronate and risedronate
30. Although the risk of MRONJ after three years of BP administration has been established
4,6, MRONJ has occurred after two years of continuous ibandronate use
30.
The results of this study and of previous studies and position papers report tooth extraction as the most common trigger of MRONJ, followed by local infection and implant procedure
5,7,8,25.
MRONJ severity staging by the AAOMS
2 rates the least severe disease as stage 0 and the most severe as stage 3; the treatment was standardized by stage in both groups as described in the treatment protocol regardless of preoperative symptoms. The preoperative symptoms determined only whether the patients should be treated immediately without discontinuation or could wait for 3 months with antibiotic and analgesic medication before surgery, such as emergency management for pathologic fracture, extraoral pus discharge, or suspected malignancy. However, in an emergency case where surgery cannot be postponed, it is highly likely that the initial degree of MRONJ was at least moderately severe. The conservative and surgical treatments for MRONJ are controversial
13. However, most of the MRONJ recurrence and progression were found after conservative treatment due to incomplete removal of the necrotic bone and its surrounding infected tissue. More extensive surgical approaches, such as sequestrectomy, saucerization, and even mandibulectomy, have been considered as the treatment choice, especially for stages 2 and 3, and have shown good prognosis and high success rates
31,32. When MRONJ affects the maxilla, especially the posterior part, the disease is often accompanied by maxillary sinusitis due to the close relationship of the maxillary teeth and alveolar bone to the maxillary sinus
33. In our study, some patients with MRONJ in the maxilla also had infection in the maxillary sinus and underwent sinus surgery concomitant with saucerization.
In this study, the number of interventions in the non-suspension group was significantly higher than in the BP suspension group (
P<0.05), accounting for up to 14 interventions in one year. Recurrence was the most common reason for additional management. In our study, suspension of BPs was associated with treatment outcome (
P<0.05); 72.2% of cases were successfully resolved and 11.1% of lesions were reduced in the suspension group. In line with previous studies
12,14, our findings showed that choice of treatment modality contributed to good prognosis and outcome of MRONJ, as did suspension of BPs before surgery.
Pathognomonic imaging features for MRONJ have not been established
34. Before computed tomography (CT) was available, panoramic radiographs were the sole means of diagnosis and prognosis. CT is superior to panoramic radiographs because the necrosis level of the cortex bone, trabecular bone density, and sequestration position can be determined accurately
21,34. However, compared to CT, panoramic radiographs offer lower costs, which is beneficial for periodic follow-up. In MRONJ, sclerotic changes were often observed. Following surgery, radiographic densities were decreased due to removal of pathological bone; during the healing period, bone density increased
35. In the present study, the relative bone density in the suspension group showed significant changes over follow-up, while the non-suspension group showed no significant changes. The changes in patients who suspended BPs were characterized by a continuous increase in bone density over time through constant bone regeneration. However, changes fluctuated in the patients who did not suspend BPs. These changes in relative density were affected by the number of interventions that patients received. When MRONJ recurred, the bone had not fully healed and was accompanied by lesion extension to an adjacent area, requiring additional surgery. Following this subsequent surgery, the bone density decreased due to further removal of the diseased bone, and additional healing time was needed to achieve significant bone density changes. Due to the minimal number of interventions in the group that suspended BPs, the bone recovered uneventfully, resulting in increased relative bone density. When comparing the BP suspension and non-suspension groups, significant relative bone density changes were found at 6-month and 1-year follow-ups after treatment. This is explained by bone remodeling and BP suspension as proposed by Damm and Jones
11. The period of bone remodeling varies from 2 to 8 months in a generally healthy person. Because remodeling is impaired in MRONJ patients, additional BP suspension after surgery for a minimum of 4 to 8 months was suggested
11. After peak bone remodeling, significant difference in bone healing evaluated through relative density was observed at 6-month and 1-year follow-ups between the two groups.
ESR is used to assess the acute phase response to inflammation because it decreases following the resolution of inflammation
36. ALP plays an integral role in metabolism within the hepatobiliary system and skeleton and has been considered a useful marker of hepatobiliary diseases, bone disorders, and wound healing
37,38. The increase in ALP is related to cellular damage
37. In previous studies, ESR and ALP serum levels were higher in the MRONJ group compared to the non-MRONJ group
39,41. According to the literature, elevated ESR in MRONJ not only reflects the ongoing process of infection to the bone necrosis site, but also progression of MRONJ
40. Elevated ALP in MRONJ was correlated with bone sclerosis with persisting alveolar socket, osteolysis, and sequestrum
39. In a previous study on MRONJ wound healing protein profile using immunoprecipitation high-performance liquid chromatography (IP-HPLC), the ALP level of postoperative exudate (POE) was lower from 6 hours to 1 day after surgery. However, 2 days after surgery, the ALP in the removed POE was increased and almost recovered to the level of the study control, indicating wound healing by removal of inflammatory toxins
38. In our study, the ESR level was not significantly increased with ALP after surgery in the non-suspension group. On the other hand, both ALP and ESR levels were significantly decreased in the BP suspension group. The ALP changes in both groups were in the normal range. To further investigate the simultaneous alteration of ESR and ALP, we performed a correlation test, and the result revealed a positive correlation between ESR and ALP. A positive correlation of ALP with other inflammatory markers, such as C-reactive protein (CRP) and leukocytes, was also found in previous studies
42,43. To our knowledge, the positive correlation between ESR and ALP in MRONJ has not been mentioned anywhere. The positive correlations between ALP and inflammatory markers were explained through an oxidative stress mechanism
43. In recent studies, BPs have been reported to cause strong oxidative stress and cellular apoptosis through overproduction of reactive oxygen species (ROS), which lead to MRONJ development
44,45. Furthermore, in a previous study using IP-HPLC on the effect of nitrogen BPs (nBPs) in macrophage cell lines, the nBP stimulated cell-mediated immunity and chronic inflammation, inhibited the wound repair processes, and increased the expression of ALP
46. This knowledge and the concept of BP suspension explain the ESR and ALP elevation in the non-drug suspension group and the reduction of these markers in the BP suspension group. When BPs are suspended for at least 3 months before surgery, the levels in the serum were extremely low, and their pharmacodynamics and toxicity to the cells decreased. Following surgery, removal of necrotic bone as the source of infection led to reduction of the inflammatory reaction; however, the inflammatory reaction still occurred due to wound healing after surgery. Resolution of infection together with the low level of BPs in the serum led to the reduction of ROS and oxidative stress, reducing ALP and ESR in the serum. However, the level of BP was high in the serum following surgery in the group that did not suspend BPs. Together with the inflammation reaction caused by surgery, the ROS and the oxidative stress were elevated, causing elevation of ESR and ALP. As mentioned before, in relation to the number of interventions, the non-drug suspension group received multiple interventions due to recurrence of the disease compared to the suspension group. In a previous study, ALP level in the MRONJ POE was a wound healing marker with potential to predict MRONJ resolution or recurrence
38. Therefore, simultaneous changes of ESR and ALP in serum can become predictors of MRONJ treatment prognosis. Furthermore, the laboratory findings of this study further strengthen the evidence that BP suspension is a prognostic factor for treatment outcomes and MRONJ resolution.
The limitations of this study are the small number of patients in the non-drug suspension group and the use of panoramic radiographs. Compared to the panoramic radiograph, CT provides more detailed characteristics of the disease, especially the sclerotic and osteolytic changes of cortical bone in MRONJ patients. However, because the availability of CTs was limited, as they were only performed preoperatively, periodic follow-up measurements could only be obtained from panoramic radiographs. Moreover, the use of more specific bone markers, such as bone-alkaline phosphatase, TRACP5b, and other inflammatory markers such as CRP, are recommended for further prospective studies.
We conducted this study retrospectively. Existing data that have been recorded for reasons other than research in the form of medical records were scrutinized and the inclusion of subjects focused on the study question to clarify the hypothesis and identify feasibility issues for a prospective study
47. However, due to a dependency on medical records, some important data might be missing from the study. The number of samples is also affected, this may result in hidden bias and is one of the disadvantages of a retrospective study
48.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download