INTRODUCTION
Ensuring high quality of laboratory tests is crucial for accurate clinical diagnosis and treatment. Laboratory test errors are divided into pre-analytical, analytical, and post-analytical errors [
1], with pre-analytical errors accounting for approximately 60%–70% of all errors [
2,
3]. The most common pre-analytical errors related to specimen management are hemolysis and clots [
4]. Poor specimen management in this phase will decrease the specimen quality and therefore impact the test results and their interpretation. For example, hemolyzed specimens can show results of elevated potassium, lactate dehydrogenase (LDH), AST, and ALT levels [
5], whereas uncentrifuged specimens preserved for prolonged periods can provide erroneous test results for AST, LDH, glucose, and calcium [
6].
Detailed standard operating procedures must be strictly followed for all laboratory processes—from ordering to specimen collection, specimen handling, and storage—to manage the quality of the pre-analytical phase. The Laboratory Accreditation Program of the College of American Pathologists in the United States and the Outstanding Laboratory Accreditation Program (OLAP) of the Laboratory Medicine Foundation (LMF) in Korea are certification programs for laboratories that check whether the appropriate procedures are followed. The International Society for Quality in Health Care approved the Korean OLAP in 2017 to improve laboratory quality and promote high-level laboratory management [
7]. However, the Korean OLAP is intended only for relatively large facilities led by clinical pathologists, and only 332 facilities participated in their accreditation in 2021.
In Korea, there are over 30,000 primary clinics [
8], the majority of which require tests to be performed in external referral laboratories. As sending a specimen to a referral laboratory delays the start time of the test, the results may be influenced by the quality of the pre-analytical phase. Clinicians who do not specialize in laboratory medicine generally have a poor understanding of the overall laboratory test process and the risks associated with incorrect laboratory results. Pre-analytical errors can be attributed to procedural errors during specimen collection, handling, and storage [
9], which clinicians can easily overlook, and there is no mandatory system for pre-analytical laboratory test quality management in primary care clinics. Although the National Health Insurance Corporation has mandated the LMF to audit quality management in facilities that carrying out the National Health Screening Program (NHSP), the effectiveness of quality assessment is limited because most procedures are limited to paper assessments, unlike OLAP, and facilities that do not carrying out the NHSP are not assessed for quality.
We aimed to understand the current state of pre-analytical quality management in laboratory medicine among primary clinics in Korea that request laboratory tests from referral laboratories. Data were collected from surveys sent to clinics across the country. This study can help emphasize the importance of pre-analytical management in clinical laboratories.
Go to :

RESULTS
A total of 141 physicians completed the survey. Approximately three quarters (108/141) of the facilities where the respondents work require that all laboratory tests be performed externally at referral laboratories. Slightly less than half of the respondents were internal medicine physicians (65, 46.1%), with the remaining being family medicine physicians. Of the 141 facilities, 73 (51.8%) carried out the NHSP. Facilities that carried out the NHSP had more internal medicine physicians (50/73, 68.5%), whereas facilities that did not carried out the NHSP had more family medicine physicians (53/68, 77.9%) (
P<0.0001).
Table 1 presents detailed characteristics of the survey respondents.
Table 1
Characteristics of the survey respondents
|
Questionnaire |
N (%) |
|
Clinic implements the National Health Screening Program |
|
|
Yes |
73 (51.8) |
|
No |
68 (48.2) |
|
Where are clinical chemistry tests performed? |
|
|
All clinical chemistry tests are performed in referral laboratories |
108 (76.6) |
|
Only clinical chemistry tests using serum specimens are performed in referral laboratories |
33 (23.4) |
|
Respondent’s specialty |
|
|
Family medicine |
76 (53.9) |
|
Internal medicine |
65 (46.1) |
|
Number of years since obtaining a medical license (yr) |
|
|
5–10 |
27 (19.1) |
|
10–20 |
78 (55.3) |
|
20 |
36 (25.5) |
|
Number of specimens sent to referral laboratories |
|
|
51–100 patients/day |
3 (2.1) |
|
11–50 patients/day |
35 (24.8) |
|
6–10 patients/day |
42 (29.8) |
|
≤ 5 patients/day |
39 (27.7) |
|
3–4 patients/week |
17 (12.1) |
|
≤ 2 patients/week |
5 (3.5) |
|
Location of referral laboratories |
|
|
Seoul |
68 (48.2) |
|
Gyeonggi |
39 (27.7) |
|
Other areas |
19 (13.5) |
|
Do not know |
15 (10.6) |

A summary of the responses to the main survey questions related to pre-analytic stages of specimen handling is provided in
Table 2. In more than 60% of the primary clinics surveyed (65.2%, 92/141), patient information was handwritten rather than barcoded on the specimen bottle. Only one piece of patient information (name or identification number) was included on the label of the specimen bottle in 14.2% of the clinics, whereas 19.9% of the respondents reported including two pieces of patient information. Clinical chemistry tests were conducted in plain tubes in 18.4% of the primary clinics, and a serum separation tube was used in 80.8% of the primary care clinics. Bottles manufactured by BD Diagnostic Systems (Sparks, MD, USA) and Greiner Bio-One GmbH (Kremsmuenster, Austria) were the most commonly used specimen bottles (75.2%), which were provided by the referral laboratories in most cases (83.7%). Lack of a centrifuge in the clinic was reported by 29.1% of the respondents. Approximately half of the primary clinics provided instructions for specimen storage (53.9%). Specimens were generally transported to the referral laboratory only once per day (86.5%) during workdays. Nearly all respondents knew that some test results would be erroneous if the blood was hemolyzed (96.5%). However, in less than half (45.1%) of the primary clinics, specimens were typically recollected and retested when hemolysis was considered to have caused errors in the results (
Table 2).
Table 2
|
Category |
Question |
N (%) |
|
Specimen bottle |
Patient information included on the bottle |
|
|
Barcoded |
49(34.8) |
|
Handwritten |
92(65.2) |
|
Number of pieces of patient information |
|
|
1 |
20(14.2) |
|
2 |
28(19.9) |
|
3 |
23(16.3) |
|
4 |
36(25.5) |
|
5 |
25(17.7) |
|
6 |
9(6.4) |
|
Collection tube for routine chemistry tests |
|
|
Plain tube |
26(18.4) |
|
SST |
114(80.9) |
|
Others |
1(0.7) |
|
Manufacturer of SST |
|
|
BD |
54(38.3) |
|
Greiner |
52(36.9) |
|
AB Medical |
12(7.5) |
|
Others |
9(6.4) |
|
Unknown |
14(9.9) |
|
How the specimen bottle is selected |
|
|
Referral laboratory supplies the specimen bottle |
118(83.7) |
|
The clinic provides the referral laboratory with the recommended collection bottle |
16(11.3) |
|
The clinic purchases collection bottles as they see fit |
6(4.3) |
|
Unknown |
1(0.7) |
|
Centrifugation |
A centrifuge is available at the clinic |
|
|
Yes |
100(70.9) |
|
No |
41(29.1) |
|
Reason for not having a centrifuge at the clinic |
|
|
Too expensive |
8(19.5) |
|
Difficult to manage |
22(53.7) |
|
Unsure of the need |
10(24.4) |
|
Unknown |
1(2.4) |
|
Period of centrifugation (when available at the clinic) |
|
|
~30 minutes after sampling |
37(37.0) |
|
Immediately after sampling |
41(41.0) |
|
At a designated time (e.g., once a day in the afternoon) |
10(10.0) |
|
Rarely use a centrifuge |
8(8.0) |
|
On arrival at the referral laboratory |
3(3.0) |
|
Others |
1(1.0) |
|
Specimen storage temperature after centrifugation |
|
|
Refrigerator temperature |
77(86.5) |
|
Room temperature |
12(13.5) |
|
Specimen storage condition after centrifugation |
|
|
In an SST |
67(58.3) |
|
In a plain tube |
24(20.9) |
|
Transfer serum into a serum separator |
15(13.0) |
|
Transfer serum into a microtube |
9(7.8) |
|
Specimen storage temperature without centrifugation |
|
|
Refrigerator temperature |
35(66.0) |
|
Room temperature |
17(32.1) |
|
Freezing temperature |
1(1.9) |
|
Specimen storage condition without centrifugation |
|
|
Insert the tube in an upright position into a rack |
41(77.4) |
|
Placed upright or tilted in paper cups, etc. |
8(15.1) |
|
Storage in a basket, etc. |
3(5.7) |
|
Unknown |
1(1.9) |
|
Specimen storage/delivery |
Do referral laboratories have trained clinic staff on specimen storage |
|
|
Trained |
76(53.9) |
|
Not trained |
64(45.4) |
|
Unknown |
1(0.7) |
|
Number of times specimens are delivered during workdays (Monday to Friday) |
|
|
2 times/day |
12(8.5) |
|
1 time/day |
122(86.5) |
|
< 1 time/day |
7(5.0) |
|
Number of times specimens are delivered on a saturday |
|
|
2 times/day |
1(0.7) |
|
1 time/day |
106(75.2) |
|
< 1 time/day |
8(5.7) |
|
None |
17(12.1) |
|
Clinic is not open |
8(5.7) |
|
Unknown |
1(0.7) |
|
Number of times specimens are delivered on a sunday |
|
|
1 time/day |
2(1.4) |
|
< 1 time/day |
3(2.1) |
|
None |
43(30.5) |
|
Clinic is closed |
93(66.0) |
|
Specimen delivery schedule the day before the clinic is closed |
|
|
All specimens are delivered the day before |
60(42.6) |
|
Specimens are delivered as scheduled and the remaining are delivered on the day the clinic opens |
15(10.6) |
|
Specimens are delivered as scheduled and the remaining are delivered at designated times on the day the clinic opens |
60(42.6) |
|
No days of clinic closure |
4(2.8) |
|
Others |
2(1.4) |
|
Specimen quality |
Awareness that some test results are erroneous when blood is hemolyzed |
|
|
I know |
136(96.5) |
|
I do not request tests that are affected by hemolysis |
3(2.1) |
|
I do not know |
2(1.4) |
|
Frequency of receiving erroneous results due to hemolysis |
|
|
~1/10 patients |
4(2.8) |
|
~1/100 patients |
26(18.4) |
|
~1/1,000 patients |
53(37.6) |
|
~1/10,000 patients |
41(29.1) |
|
Almost never |
17(12.1) |
|
If hemolysis is thought to have caused an erroneous result, the following actions are taken |
|
|
Resampling and retesting |
79(45.1) |
|
If the test is not critical, no further testing |
42(24.0) |
|
Inform the patient that no resampling will be conducted |
26(14.9) |
|
Retest using the previous specimen |
20(11.4) |
|
Do not disclose to the patient; no resampling conducted |
4(2.3) |
|
Others |
4(2.3) |

Table 3 compares the survey responses for clinics grouped according to whether or not they implement the NHSP. Institutions implementing the NHSP most frequently used a barcode system rather than another system (52.1% vs. 16.2%,
P<0.0001). There was no significant difference in the amount of patient information and type of routine chemistry test bottles used between clinics that do and do not implement the NHSP. The proportion of primary clinics with centrifuges was higher among those that implement the NHSP than among those that do not (83.6% vs. 57.4%,
P<0.0006). However, there were no significant differences in the responses related to centrifuge condition questions, including the timing of centrifugation, temperature, and post-centrifugation storage conditions, between clinics that do and do not implement the NHSP. The numbers of specimens delivered on Saturday, Sunday, or the day before a holiday were not significantly different between the two groups (
P=0.10, 0.19, and 0.62, respectively). The responses to questions related to the procedures for hemolyzed specimens were not significantly different between the two groups (
Table 3 and
Fig. 1).
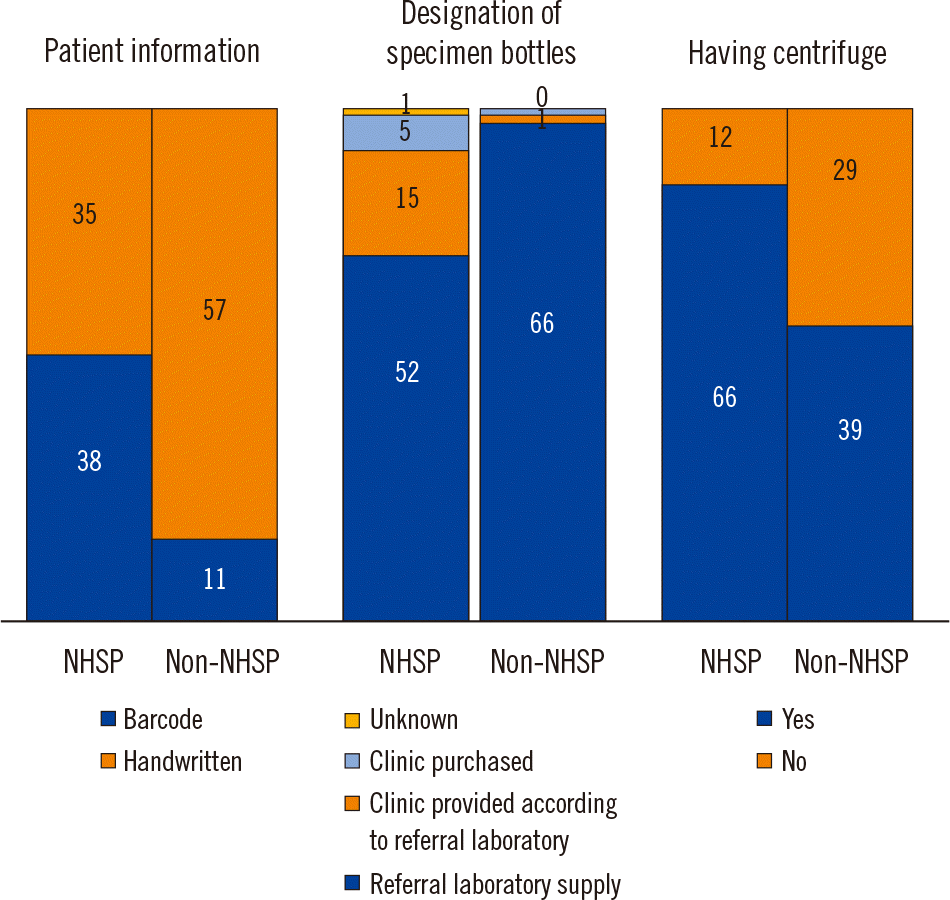 | Fig. 1Differences between primary clinics that do and do not implement the National Health Screening Program (NHSP). 
|
Table 3
Comparison of responses based on whether the NHSP is implemented
|
Category |
Question |
Institutions that implement the NHSP (N=73), N (%) |
Institutions that do not implement the NHSP (N=68), N (%) |
P
|
|
Specimen bottle |
Patient information included on the bottle |
|
|
< 0.0001 |
|
Barcoded |
38 (52.1) |
11 (16.2) |
|
|
Handwritten |
35 (47.9) |
57 (83.8) |
|
|
Bottle used for routine chemistry tests |
|
|
0.87 |
|
Plain tube |
13 (17.8) |
13 (19.1) |
|
|
SST |
59 (80.8) |
55 (80.9) |
|
|
Others |
1 (1.4) |
0 (0.0) |
|
|
Factors that determine the specimen tube used |
|
|
0.0003 |
|
Referral laboratory supplies the specimen collection tube |
52 (71.2) |
66 (97.1) |
|
|
Clinic provides referral laboratory-recommended bottle |
15 (20.5) |
1 (1.5) |
|
|
Clinic purchases the bottles as they see fit |
5 (6.8) |
1 (1.5) |
|
|
Unknown*
|
1 (1.4) |
0 (0.0) |
|
|
Centrifugation |
A centrifuge is available at the clinic |
|
|
0.0006 |
|
Yes |
61 (83.6) |
39 (57.4) |
|
|
No |
12 (16.4) |
29 (42.6) |
|
|
When is centrifugation conducted (if the clinic has an on-site centrifuge) |
|
|
0.86†
|
|
~30 minutes after sampling |
23 (37.7) |
14 (35.9) |
|
|
Immediately after sampling |
27 (44.3) |
14 (35.9) |
|
|
At a designated time (e.g., once a day in the afternoon) |
7 (11.5) |
3 (7.7) |
|
|
Rarely use a centrifuge |
2 (3.3) |
6 (15.4) |
|
|
On arrival at the referral laboratory |
2 (3.3) |
1 (2.6) |
|
|
Others |
0 (0.0) |
1 (2.6) |
|
|
Specimen storage/delivery |
Specimen storage temperature after centrifugation |
|
|
0.40 |
|
Refrigerator temperature |
48 (84.2) |
29 (90.6) |
|
|
Room temperature |
9 (15.8) |
3 (9.4) |
|
|
Specimen storage condition after centrifugation |
|
|
0.53‡
|
|
In an SST |
38 (57.6) |
29 (59.2) |
|
|
In a plain tube |
12 (18.2) |
12 (24.5) |
|
|
Transfer serum into a serum separator |
9 (13.6) |
6 (12.2) |
|
|
Transfer serum into a microtube |
7 (10.6) |
2 (4.1) |
|
|
Specimen storage temperature without centrifugation |
|
|
0.08 |
|
Refrigerator temperature |
8 (50.0) |
27 (73.0) |
|
|
Room temperature |
8 (50.0) |
9 (24.3) |
|
|
Freezing temperature§
|
0 (0.0) |
1 (2.7) |
|
|
Specimen storage condition without centrifugation |
|
|
0.39ll
|
|
Upright in a test tube rack |
14 (87.5) |
27 (73.0) |
|
|
Placed upright or tilted in paper cups, etc. |
1 (6.3) |
7 (18.9) |
|
|
Storage in a basket, etc. |
1 (6.3) |
2 (5.4) |
|
|
Unknown |
0 (0.0) |
1 (2.7) |
|
|
Whether a guide on specimen storage has been received from the referral laboratory |
|
|
0.14 |
|
Received |
44 (60.3) |
32 (47.1) |
|
|
Not received |
29 (39.7) |
35 (51.5) |
|
|
Unknown¶
|
0 (0.0) |
1 (1.5) |
|
|
I do not know |
2 (2.7) |
0 (0.0) |
|
|
Specimen quality |
Frequency of experiencing hemolysis that causes erroneous results |
|
|
|
|
~1/10 patients |
1 (1.4) |
3 (4.4) |
0.09**
|
|
~1/100 patients |
11 (15.1) |
15 (22.1) |
|
|
~1/1,000 patients |
36 (49.3) |
17 (25.0) |
|
|
~1/10,000 patients |
16 (21.9) |
25 (36.8) |
|
|
Almost never |
9 (12.3) |
8 (11.8) |
|
|
If hemolysis is believed to have caused an erroneous result, the following actions are taken |
|
|
0.76††
|
|
Resampling and retesting |
43 (46.2) |
36 (38.7) |
|
|
If the test is not critical, no further action is taken |
19 (20.4) |
23 (24.7) |
|
|
Inform the patient that no resampling will be conducted |
17 (18.3) |
9 (9.7) |
|
|
Retest using the previous specimen |
12 (12.9) |
8 (8.6) |
|
|
Do not disclose to the patient; no resampling done |
2 (2.2) |
2 (2.2) |
|
|
Others |
0 (0.0) |
4 (4.3) |
|

Categorization of the clinics according to those requesting an average of ≥6 or ≤5 specimens per day revealed differences in patient information, tube determination, the presence of a centrifuge, and specimen storage temperature (
Table 4).
Table 4
Comparison of responses based on the size of the laboratory test service
|
Category |
Question |
Institutions carrying out ≥6 tests/day (N=80), N (%) |
Institutions carrying out ≤5 tests/day (N=61), N (%) |
P
|
|
Specimen bottle |
Patient information included on the bottle |
|
|
0.0001 |
|
Barcoded |
39 (48.8) |
10 (16.4) |
|
|
Handwritten |
41 (51.3) |
51 (83.6) |
|
|
Bottle for routine chemistry tests |
|
|
0.5618 |
|
Plain tube |
16 (20.0) |
10 (16.4) |
|
|
SST |
63 (78.8) |
51 (83.6) |
|
|
Others |
1 (1.3) |
0 (0.0) |
|
|
Factors that determine the specimen tube used |
|
|
0.0325 |
|
Referral laboratory supplies the specimen collection tube |
61 (76.3) |
57 (93.4) |
|
|
Clinic provides referral laboratory-recommended tubes |
13 (16.3) |
3 (4.9) |
|
|
Clinic purchases bottles as they see fit |
5 (6.3) |
1 (1.6) |
|
|
Unknown*
|
1 (1.3) |
0 (0.0) |
|
|
Centrifuge |
A centrifuge is available at the clinic |
|
|
0.0497 |
|
Yes |
62 (77.5) |
38 (62.3) |
|
|
No |
18 (22.5) |
23 (37.7) |
|
|
When is centrifugation conducted (if the clinic has an on-site centrifuge) |
|
|
0.0847†
|
|
~30 minutes after sampling |
27 (33.8) |
10 (16.4) |
|
|
Immediately after sampling |
25 (31.3) |
16 (26.2) |
|
|
At a designated time (e.g., once a day in the afternoon) |
6 (7.5) |
4 (6.6) |
|
|
Rarely use a centrifuge |
2 (2.5) |
6 (9.8) |
|
|
On arrival at the referral laboratory |
1 (1.3) |
2 (3.3) |
|
|
Others |
1 (1.3) |
0 (0.0) |
|
|
Specimen storage/delivery |
Specimen storage temperature after centrifugation |
|
|
0.0468 |
|
Refrigerator temperature |
48 (81.4) |
29 (96.7) |
|
|
Room temperature |
11 (18.6) |
1 (3.3) |
|
|
Specimen storage condition after centrifugation |
|
|
0.1854‡
|
|
In an SST |
44 (62.0) |
23 (52.3) |
|
|
In a plain tube |
12 (16.9) |
12 (27.3) |
|
|
Transfer serum into a serum separator |
9 (12.7) |
6 (13.6) |
|
|
Transfer serum into a microtube |
6 (8.5) |
3 (6.8) |
|
|
Specimen storage temperature without centrifugation |
|
|
0.2028 |
|
Refrigerator temperature |
12 (54.5) |
23 (74.2) |
|
|
Room temperature |
9 (40.9) |
8 (25.8) |
|
|
Freezing temperature§
|
1 (4.5) |
0 (0.0) |
|
|
Specimen storage condition without centrifugation |
|
|
0.0942ll
|
|
Upright in a test tube rack |
19 (86.4) |
22 (71.0) |
|
|
Placed upright or tilted in paper cups, etc. |
2 (9.1) |
6 (19.4) |
|
|
Storage in a basket, etc. |
0 (0.0) |
3 (9.7) |
|
|
Unknown |
1 (4.5) |
0 (0.0) |
|
|
Whether a guide to specimen storage has been received from the referral laboratory |
|
|
0.0248 |
|
Received |
50 (62.5) |
26 (42.6) |
|
|
Not received |
30 (37.5) |
34 (55.7) |
|
|
Unknown¶
|
0 (0.0) |
1 (1.6) |
|
|
Specimen quality |
Frequency of experiencing hemolysis that causes erroneous results |
|
|
|
|
~1/10 patients |
2 (2.5) |
2 (3.3) |
0.1786**
|
|
~1/100 patients |
11 (13.8) |
15 (24.6) |
|
|
~1/1,000 patients |
38 (47.5) |
15 (24.6) |
|
|
~1/10,000 patients |
20 (25.0) |
21 (34.4) |
|
|
Almost never |
9 (11.3) |
8 (13.1) |
|
|
If hemolysis is believed to have caused an erroneous result, the following actions are taken |
|
|
0.2554††
|
|
Resampling and retesting |
50 (62.5) |
29 (47.5) |
|
|
If the test is not critical, no further action is taken |
22 (27.5) |
20 (32.8) |
|
|
Inform the patient that no resampling will be conducted |
13 (16.3) |
13 (21.3) |
|
|
Retest using the previous specimen |
15 (18.8) |
5 (8.2) |
|
|
Do not disclose to the patient; no resampling done |
2 (2.5) |
2 (3.3) |
|
|
Others |
0 (0.0) |
3 (4.9) |
|

Go to :

DISCUSSION
We surveyed primary care clinics across Korea to determine the current status of pre-analytical quality management of specimens sent to referral laboratories for tests. A similar study was published in 2021 by Chong,
et al. [
15]. However, they only targeted clinics on Jeju Island, and all participating facilities used the same referral laboratory; therefore, the results cannot be considered representative of the overall situation of primary care clinics in Korea. While physicians from all specialties participated in the previous study [
15], we distributed the questionnaire only to internal medicine and family medicine physicians because they order the most laboratory tests, and therefore, their decisions are the most likely to be influenced by the quality of laboratory test results. Excluding clinicians from other specialties helps to better reflect the real impact of the quality of laboratory test results on the quality of care that physicians provide to patients in their clinics. We also developed a more specific questionnaire about the pre-analytical phase focusing on aspects that could be the most problematic in practical and clinical settings, including centrifugation and blood collection.
In our study, 29.1% of respondents did not perform centrifugation at their clinics. This would be acceptable if all specimens were sent to the laboratory and analyzed immediately. Specimens that have not been centrifuged and are stored for a long time can yield erroneous laboratory results. In particular, specimens collected before weekends or holidays are often left in an uncentrifuged state for up to two days. We found that specimens from more than 50% of clinics where centrifuges were unavailable were only centrifuged upon delivery at the referral laboratory. Poor specimen quality such as hemolysis can only be detected by comparing the color of the specimens after centrifugation [
16]. Therefore, clinicians cannot determine the quality of specimens (hemolyzed or not) unless the facilities have their own centrifuges. Clinicians’ knowledge of whether patients’ specimens have been hemolyzed is essential for interpreting test results. Even in primary clinics that do use a centrifuge, the centrifugation time or specimen storage temperature after centrifugation is often inappropriate. Given this situation, it is necessary to create a mandatory training program suitable for primary care clinics. Cartoons and video-based data are easy and concise to convey the importance of this pre-analytical process. It is also essential to promote the clinician training program to uphold these standards.
Patient information was barcoded in less than half of the primary clinics surveyed (34.8%) (
Table 2); the majority of specimens was manually labeled. According to CLSI GP33 ED2 [
10], the barcode label should include, at minimum, the patient’s name and patient identifier. If handwritten, the label should include the patient’s name, patient identifier, date of specimen collection, and information regarding the collection technician [
10]. In our survey, 65.2% of the primary clinics did not meet this criterion. Handwriting on the specimen tube in the clinic takes a long time, and it is difficult to include all the information required in the CLSI guidelines on the small label; therefore, primary clinics should try to implement barcode systems whenever possible. Because most clinics do not have an on-site laboratory and send patient specimens to referral laboratories, it is possible that specimens are switched during delivery. A specimen identification error leads to a mismatch of the test result and specimen, resulting in incorrect diagnosis and treatment, posing a significant patient safety concern. Therefore, the use of a barcode on the specimen tube is recommended. If barcodes are unavailable and patient information has to be handwritten, at least three pieces of identifying information (e.g., name, hospital identification number, and age) should be included.
Barcode systems for laboratory tests are easy to implement in hospitals that use electronic health records (EHRs). Therefore, the low rate of barcode use found in this study is related to the overall low rate of EHR implementation at primary care clinics in Korea. In the United States, a national “meaningful use” project offered incentives to facilities to implement EHRs, resulting in a significant increase in the rate of EHR uptake in primary care clinics [
17,
18]. EHR adoption has been delayed in Korea because it is expensive [
19,
20]. The Logical Observation Identifiers Names and Codes (LOINC) provides a database of international standards for identifying health measurements, observations, and documents [
21]. If the LOINC code is introduced in all laboratories in Korea in the future, along with the mandatory use of barcodes, errors in specimen exchange and the transmission of laboratory test orders from primary clinics to referral laboratories can be significantly reduced.
Overall, we found that pre-analytical quality in primary clinics before specimen transport to referral laboratories is inadequate. Before distributing the questionnaires, we assumed that pre-analytical management would be better in primary clinics that implement the NHSP than in primary clinics that do not because the former facilities are subject to a paper audit by the LMF. Some questionnaire items demonstrated clear differences between primary clinics that do and do not implement the NHSP, including the use of barcodes, designation of specimen bottles, and centrifugation (
Fig. 1). However, other questionnaire items showed inadequate pre-analytical quality management in both groups of clinics (
Table 3). A limitation of the NHSP–LMF audit is that it uses a scoring system and a paper evaluation. For example, the NHSP laboratory certification checklist asks whether the laboratory has a centrifuge. While 16.4% of the clinics that implement the NHSP indicated that they do not have a centrifuge, they still received certification by the LMF because they achieved the necessary score (>60 points) based on their responses to other questions. As this is a paper evaluation based on self-assessment data, it is likely that the clinics gave themselves a generous score and thus obtained their certification.
Significant differences were observed between clinics based on the number of specimens requested per day. Clinics requesting ≥6 specimens per day performed better than those requesting ≤5 specimens per day in terms of accurate patient information, tube determination, centrifugation, and specimen storage temperature (
Table 4).
This study had some limitations. First, the total number of laboratory tests ordered per clinic was an optional question and therefore was not included in the analysis. This was because we did not aim to evaluate laboratory error rates but to gain an overall understanding of clinicians’ perceptions and the quality of the clinic’s pre-analytical phase. Second, although this was a nationwide study among members of the Korean Society of Medical Health Screening, most survey respondents were from the Seoul metropolitan area, with 37.6% from Gyeonggi Province and 42.6% from Seoul. As the number of respondents from other areas was relatively small, region-based analysis was difficult. In addition, the distance between the clinic and referral laboratory will affect the laboratory test quality more than the geographical location of the clinic itself. However, owing to the limitations of the survey, this factor was not included in this study.
This was the first study to administer a questionnaire to clinicians from across Korea regarding the pre-analytical quality management of laboratory tests sent to referral laboratories. Laboratory test results are used not only by primary clinics but also by specialists in large hospitals for examination and treatment decisions. Moreover, test results can be included in broader research studies. Therefore, like in tertiary hospitals, the quality of laboratory test results in primary clinics must be guaranteed. Most studies on pre-analytical phase management have been conducted at above the general hospital level, where clinical pathologists work in on-site laboratories [
4,
5]. This was the first study to focus on clinicians’ awareness and the quality of facilities and instruments in primary clinics. Based on our results, we suggest expanding pre-analytical phase management to these clinics. Apart from providing accurate primary data, we believe that factors outside the laboratory should be considered to ensure the accuracy of test results. An appropriate management system will need to be established to enable proper education, public promotion, and quality management in the pre-analytical phase in primary care clinics.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download