Abstract
Background
Sterility and safety assurance of hematopoietic stem cell (HSC) products is critical in transplantation. Microbial contamination can lead to product disposal and increases the risk of unsuccessful clinical outcomes. Therefore, it is important to implement and maintain good practice guidelines and regulations for the HSC collection and processing unit in each hospital. We aimed to share our experiences and suggest strategies to improve the quality assurance of HSC processing.
Methods
We retrospectively analyzed microbial culture results of 11,743 HSC products processed over a 25-year period (January 1996 to May 2021). Because of reorganization of the HSC management system in 2008, the 25-year period was divided into periods 1 (January 1996 to December 2007) and 2 (January 2008 to May 2021). We reviewed all culture results of the HSC products and stored aliquot samples and collected culture results for peripheral blood and catheter samples.
Results
Of the 11,743 products in total, 35 (0.3%) were contaminated by microorganisms, including 19 (0.5%) of 3,861 products during period 1 and 16 (0.2%) of 7,882 products during period 2. Penicillium was the most commonly identified microorganism (15.8%) during period 1 and coagulase-negative Staphylococcus was the most commonly identified (31.3%) during period 2. HSC product contamination occurred most often during HSC collection and processing.
Conclusions
The contamination rate decreased significantly during period 2, when the HSC management system was reorganized. Our results imply that handling HSC products by trained personnel and adopting established protocols, including quality assurance programs, aid in decreasing the contamination risk.
Hematopoietic stem cells (HSCs) are widely used as hematopoietic progenitors for autologous transplantation in patients with malignant diseases [1]. Over the last five decades, more than one million HSC transplantations (HSCTs) using HSCs from various sources (bone marrow [BM], mobilized peripheral blood, and umbilical cord blood) have been performed [2].
The preparation of HSCs for transplantation involves multiple steps, including the collection, processing, testing, preservation, storage, and distribution of products [3, 4]. These steps are entrusted to cell therapy laboratories and stem cell banks. The quality, traceability, and safety of HSC products must be ensured [5]. At each step during HSC collection and processing, there is a risk of product contamination. Contaminated HSC products can cause infections when used in stem cell transplantation therapies [6].
Keeping HSC products sterile is crucial for successful and safe transplantation. Controlling microbial contamination in HSC products is one of the most important issues in terms of QC [7, 8]. Although culture-positive HSC products do not necessarily cause serious infection in recipients, microbial contamination of HSC products often leads to arrest of or delay in the treatment plan [9-11].
However, a positive blood culture result does not necessarily indicate contamination of the product itself. There are various culture contamination sources, including the product itself, phlebotomy preparation, laboratory tools or apparatuses, including storage bags and pipettes, the medium, culture bottle, people, and the air [12]. Therefore, it is important to determine the source of microbial contamination not only for quality assurance but also for processes ranging from decision-making to infusion, because discarding the product results in a shortage of transplantation material, which is detrimental to clinical outcomes.
The Samsung Medical Center is one of the largest tertiary care hospitals in Seoul, Korea, where various transplantation programs, including HSCTs, are performed. The center implemented HSCT in 1996. In 2008, the Cellular Therapeutics Laboratory (CTL), which was responsible for various tasks, ranging from HSC product processing to distribution, was reorganized within the Department of Laboratory Medicine and Genetics. Through this reorganization, collaboration among collection, processing, storage, and clinical laboratories for quality measurement became possible. Moreover, standardized operating procedures and computerized laboratory information systems (LIS), ranging from HSC collection to distribution, and a product safety monitoring strategy were established. We retrospectively reviewed the contamination rate of peripheral blood stem cell (PBSC) products collected in a single center over a 25-year period (January 1996 to May 2021) and determined the causes of contamination to develop strategies for improving the quality assurance of HSC processing.
Between January 1996 and May 2021, 11,743 HSC products were collected from 3,179 patients (donors) and cryopreserved at the Samsung Medical Center. The major product type was peripheral blood (99.8%), the other product type was BM. Cord blood was not included in this study because cord blood products were not collected or processed at our center. This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB approval number: 2022-12-126).
In 2008, the HSC processing and storage units, which previously belonged to the Hemato-Oncology Clinical Department, were reorganized into a single, separate unit, termed CTL, within the Department of Laboratory Medicine and Genetics. An entire process management system, including a computerized LIS facility, was developed and implemented. Details are presented in Table 1. Therefore, the 25-year period was divided into two periods: January 1996 to December 2007 (period 1) and January 2008 to May 2021 (period 2).
All products were collected in the apheresis unit, using a sterile technique. Peripheral blood products were collected from autologous or allogeneic donors via a subclavian catheter or from the peripheral vein by apheresis using COBE Spectra and Spectra Optia (Terumo Blood and Cell Technologies, Lakewood, CO, USA). They were then transferred to the CTL. BM was harvested from the posterior iliac crest by a physician in the operation room. CTL staff assisted in sample collection by adding the anticoagulant ACD-A (BM:ACD-A=24:1) to the collection bags and then transferred the samples to the CTL for further processing. All autologous products were cryopreserved after collection, whereas most allogeneic products were infused into recipients without further manipulation or cryopreservation. Allogeneic products were cryopreserved only in certain circumstances, e.g., when the recipient’s condition deteriorated or an unexpected fever developed.
For cryopreservation, the products were centrifuged (4,000×g for 5 minutes) and the number of cells was adjusted to 4-6×108/mL after plasma extraction. The concentrated products were mixed with a freezing solution (60% Medium 199, 20% autologous plasma or albumin, 20% dimethyl sulfoxide, heparin 10 units/mL) at a 1:1 ratio for cryopreservation. The final cell concentration for cryopreservation was 2-3×108/mL. Samples were collected for complete blood count, CD34+ count (using flow cytometry), and subset analysis (using flow cytometry) [13].
From 2008 onwards, for each collected product, three aliquots were prepared in the preparation step to evaluate HSC product quality. Each aliquot contained approximately 500 µL of HSC product mixed with freezing solution. They were cryopreserved in the same manner as the product bags. The aliquots were used for viability tests, estimation of cellular components, clonogenic assays, or microbial culture if product contamination was suspected.
To evaluate the sterility of a product, samples for microbial culture were collected from the product immediately before cryopreservation. Different culture methods were used in periods 1 and 2. In period 1, samples for microbial tests were collected from HSC products using sterile smear loops (10 µL) and inoculated onto blood agar plates that were incubated at 37°C in a 5% CO2 incubator overnight. In period 2, plasma samples (4 mL) containing residual cellular components were collected by rinsing the emptied bag of admixed HSC product and freezing solution with 5-10 mL of autologous plasma. The samples included several potential sources of contamination, including the autologous plasma supernatant of the HSC product, a remnant of the cellular component inside the bag, and the freezing solution. Each plasma sample was inoculated into a BacT/Alert PF pediatric blood culture bottle (bioMérieux, Marcy l’Étoile, France) and incubated in a BacT/ALERT 3D or BacT/Alert Virtuo (bioMérieux) incubator for five days.
Microbial culture results for all HSC products in periods 1 and 2 were analyzed. We reviewed the medical histories of the patients and donors whose HSC products showed positive culture results in the sterility tests. We compared the microbial species identified with the culture results of blood drawn via a central intravenous catheter or from a peripheral vein, other clinical samples, and aliquots if available. Based on the results, we defined potential source(s) or step(s) of contamination as collection/processing, culture, bacteremia, catheter, or undefined. In addition, we evaluated the clinical course of recipient patients who had undergone transplantation with contaminated products under the discretion of a physician.
Among 11,743 products collected from 3,179 patients and donors during the 25-year period, 35 (0.3%) yielded positive microbial culture results. The numbers of annually collected HSCs and proportion of culture-positive products are shown in Fig. 1. The highest contamination rate of 1.7% was observed in 2001. The number of HSCs was increased in period 2 compared to that in period 1. However, the proportion of contaminated products decreased from 0.5% (19 out of 3,861 products in period 1) to 0.2% (16 out of 7,882 products in period 2). The contaminated products included 32 products from autologous PBSC collection from patients and three products from allogeneic PBSC collection from donors. The microbial species identified and type of contamination according to the time period are presented in Table 2. Among the 19 contaminated products in period 1, three products (15.8%) were contaminated with Penicillium, which was the most frequently identified contaminant in this period. In the other nine products, the microorganisms identified all differed. Among the 16 contaminated products in period 2, coagulase-negative Staphylococcus (CoNS) was identified in four (25.0%) products and was the most frequently identified contaminant in this period.
The source of contamination was determined by identifying the culture results of patient blood collected from a peripheral vein or via a catheter, other samples, such as body fluids, and HSC aliquots (Table 2). In period 1, nine products were contaminated during the collection/processing procedure because simultaneously performed peripheral and/or catheter-drawn blood cultures were negative. The contamination sources in the other 10 cases were undefined because of the lack of other culture results.
During period 2, eight aliquot samples were additionally cultured to identify the source of contamination; in period 1, stored aliquot samples were not available to identify the microbial contamination source. Among the 16 contaminated products, eight (50.0%) yielded positive microbial culture results from other specimens (six bacteremia- and two catheter-associated positive culture results). For one culture-positive product, peripheral and catheter blood microbial culture results on the same day were negative; however, Escherichia coli, the same microorganism identified in the HSC product, was isolated from urine and aliquots. Six products were classified as having been contaminated during the culture procedure because repeated culture results of stored aliquots were negative. One product was suspected of having been contaminated during collection. For one product, the source of contamination could not be determined.
Four microbial culture-positive HSC products were infused into patients based on the physician’s decision (Table 3). The stored products were considered not to be contaminated. If a product was culture-positive, the contamination was considered to have occurred during the culture procedure. In period 1, one autologous PBSC product in which Pseudomonas aeruginosa was identified was infused. The contamination was suspected to have occurred during the collection process. The patient infused with this product was diagnosed as having neuroblastoma and initially treated with the prophylactic antibiotic teicoplanin, followed by ticarcillin/clavulanic acid and gentamicin one day after transplantation. In period 2, three contaminated products (one allogeneic BM product and two allogeneic PBSC products) were infused (Table 3). In the contaminated BM product, Corynebacterium jeikeium was identified, and the contamination was suspected to have occurred during the culture process. The patient was diagnosed as having severe aplastic anemia, and the prophylactic antibiotics acyclovir, cefepime, teicoplanin, and micafungin were administered. In the allogeneic PBSC products, Leuconostoc pseudomesenteroides and Streptococcus salivarius were identified. Both patients were diagnosed as having AML, and prophylactic antibiotics were administered. One patient was treated with acyclovir, cefepime, and micafungin; the other received acyclovir and voriconazole. None of the contaminated HSC product recipients experienced any symptoms or showed signs of bacteremia or sepsis within 24 hours after infusion (Table 3).
We analyzed microbial contamination rates of HSC products over a 25-year period divided into two periods and examined factors that may affect the performance of the CTL. The microbial contamination rate of HSC products in our hospital was 0.5% in period 1 (1997-2007). In period 2 (2008-2021), the contamination rate decreased to 0.2%, despite the increase in blood culture procedures. Tracing the sources of microbial contamination by adopting aliquot examination revealed that patient-derived contamination was frequent.
According to previous studies, microbial contamination rates vary from 0.2% to 4.5% [9, 14-16]. We observed a contamination rate of 0.3% in the 25 years from 1997 to 2021, which is low compared to the previously reported rates. Furthermore, the contamination rate was lower in period 2 (0.2%) than in period 1 (0.5%). Considering that inoculating a 4-mL plasma sample into a blood culture bottle with a five-day incubation period (period 2) likely is a more sensitive sterility test than inoculating 10 µL of HSC product onto a blood agar plate with overnight incubation (period 1), the lower contamination rate in period 2 is even more substantial.
Standards and accreditation issued by the Foundation for the Accreditation of Cellular Therapy (FACT) (https://factglobal.org/) are currently widely accepted and implemented in cellular therapies. The FACT has released several standards, including the “JACIE” international standards for hematopoietic cellular therapy regarding the collection, processing, and administration of HSC products as well as hematopoietic cellular components. They have greatly aided in transplantation organization in many institutes [17]. Because of the paucity of national guidelines or regulations for HSC processing and quality assurance systems, the Korean Society of Apheresis conducted a voluntary surveillance of HSC collection and processing status through the establishment of an HSC registry (www.ksfa-registry.org) between 2015 and 2018. According to the surveillance results for 19 out of the 30 institutes that perform HSC processing in Korea, HSC processing units were operating in variable conditions in terms of personnel, operating systems, and facilities [18]. In our hospital, the HSC processing and storage units were reorganized into a single, separate unit, the CTL, within the Department of Laboratory Medicine and Genetics. Several substantial changes were observed between periods 1 and 2. These changes have contributed to the improvement in the overall performance of HSC processing from collection to distribution, eventually lowering the contamination rate. All changes have contributed to the improvement in the quality of operation. Most importantly, the cell processing and storage units were integrated into an independent clinical laboratory-based unit. The CTL not only handles HSC products but also integrates laboratory tests to qualify products from collection to infusion. An independent processing and cryopreservation facility was established. Standard operation procedures for the entire laboratory operation, including adhering to aseptic rules, good laboratory practice, and continuous laboratory staff education, were implemented. For quality assurance, we stored aliquot samples from each product and started using an automated liquid blood culture system with a pediatric enriched blood culture bottle for contaminant identification. We believe the reduction in the contamination rate in period 2 to be owing to this improved operation system.
CoNS was the most commonly (4/35; 11.4%) isolated microorganism among culture-positive HSC products, in line with previous findings [9, 19, 20]. Penicillium spp. were detected three times and were the second most commonly isolated microorganism in the present study. Enterobacter cloacae and Staphylococcus aureus were each detected twice. The most frequently isolated microorganisms in aseptic conditions were those of the normal human skin flora (e.g., Staphylococcus spp., Micrococcus spp., and Corynebacterium spp.). Occasionally, airborne fungal spores (e.g., Aspergillus niger and Penicillium spp.) were found. Gram-negative bacteria (e.g., Enterobacter cloacae) were detected most infrequently [21]. The possibility of microbial contamination by anaerobic bacteria could not be excluded as we did not perform anaerobic cultures during period 1 or period 2; however, anaerobic bacteria were not isolated from blood or catheter cultures during periods 1 and 2. Most of the microorganisms identified in this study are inhabitants of the normal human skin or microbiota and the environment. Under normal conditions, they generally do not cause serious diseases. However, in immunocompromised hosts, they can occasionally cause bacteremia or septicemia [22, 23].
As for the sources of HSC contamination, we were able to determine the process step in which microbial contamination occurred the most frequently during the entire HSC process. The distribution of contaminations that occurred in the different steps differed between periods 1 and 2. In period 1, the main sources of contamination were sample collection or undefined. In period 2, the main sources of contamination were bacteremia and culture procedures, including sample inoculation, followed by catheter-associated contamination. This type of contamination has been reported previously, although the technicians were well-trained [6, 24]. This indicates that following a sterile strategy based on a written protocol is crucial in each step.
Two cases of HSC contamination were identified as catheter-associated contaminations. Microbial colonization of a catheter insertion site increases the risk of bloodstream infection and has been associated with thrombosis [25]. Therefore, maintaining an aseptic state of the catheter is important not only for reducing HSC contamination but also for the patient’s health [26]. Chlorhexidine disinfection reportedly is effective in reducing the risk of catheter-associated infections [27].
The use of stored aliquots is important for tracking microbial contamination sources by comparing culture results for HSC products, blood, or body fluid samples of patients. Our results showed that the number of undefined sources (or steps) of HSC product contamination dramatically decreased from period 1 to period 2. This suggests that a cross-check strategy should be used when the initial blood culture is positive to help identify problems and save products for transplantation.
Most of the contaminated HSC products were discarded because of concerns regarding infection-related morbidity or mortality. Only four HSC products that were culture-positive but considered not to have been contaminated themselves were infused. None of the patients, including the one who was transfused with a bacteremia-associated contaminated HSC product, experienced sepsis or death due to infusion of the culture-positive HSC product. Patients experiencing fever or other side effects due to sepsis after contaminated HSC transplantation are rare [9, 28-30]. A case of febrile neutropenic attack after transplantation of contaminated HSCs has been reported [31]. Therefore, the decision to infuse a culture-positive HSC product must be made in the context of the risk associated with transplantation [32, 33]. Nevertheless, a verification process to ensure the sterile status of HSC products through routine culture is recommended for quality management in the laboratory.
This study revealed a remarkable decrease in the contamination rate after the reorganization of the CTL. We demonstrated the advantages of sterility tests using blood culture bottles and storing aliquot samples for tracking the sources of HSC contamination. Based on our findings, we conclude that the entire process of HSC product handling, ranging from collection to distribution, should be managed with established protocols, including quality assurance programs, in appropriate facilities and by trained personnel.
Notes
AUTHOR CONTRIBUTIONS
Ko YK and Lee JK analyzed the data and wrote the manuscript. Park HK performed cell processing/storage and analyzed the data. Choi KM, Han AK, Mun SK, and Park HJ performed the cell processing/storage. Choung HK and Kim SM performed apheresis to collect HSCs. Lee NY performed microbial cultures and verified the results. Kim DW and Cho D supervised the apheresis process. Kang ES designed and managed the study, supervised and discussed the results, and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
REFERENCES
1. Gratwohl A. 2002; The role of the EBMT activity survey in the management of hematopoietic stem cell transplantation. European Group for Blood Marrow Transplantation. Int J Hematol. 76(S1):386–92. DOI: 10.1007/BF03165290. PMID: 12430888.

2. Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. 2015; One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2:e91–100. DOI: 10.1016/S2352-3026(15)00028-9. PMID: 26687803.

3. Cobo F, Stacey GN, Hunt C, Cabrera C, Nieto A, Montes R, et al. 2005; Microbiological control in stem cell banks: approaches to standardisation. Appl Microbiol Biotechnol. 68:456–66. DOI: 10.1007/s00253-005-0062-2. PMID: 16012832. PMCID: PMC7080164.

4. Matuszak P, Bembnista E, Kubiak A, Kozlowska-Skrzypczak M. 2016; Liquid storage of hematopoietic stem cells versus proliferative potential colony-forming unit granulocyte-monocytes: validation of cell processing. Transplant Proc. 48:1810–3. DOI: 10.1016/j.transproceed.2016.03.010. PMID: 27496497.

5. Gianassi S, Bisin S, Bindi B, Spitaleri I, Bambi F. 2010; Risk analysis of hematopoietic stem cell transplant process: failure mode, effect, and criticality analysis and hazard analysis critical control point methods integration based on guidelines to good manufacturing practice for medicinal product ANNEX 20 (February 2008). Transplant Proc. 42:2252–3. DOI: 10.1016/j.transproceed.2010.05.041. PMID: 20692457.

6. Arlt N, Rothe R, Sielaff S, Juretzek T, Peltroche H, Moog R. 2018; Sterility release testing of peripheral blood stem cells for transplantation: impact of culture bottles and incubation temperature. Transfusion. 58:2918–23. DOI: 10.1111/trf.14910. PMID: 30260478.

7. Majado MJ, García-Hernández A, Morales A, González C, Martínez-Sánchez V, Menasalvas A, et al. 2007; Influence of harvest bacterial contamination on autologous peripheral blood progenitor cells post-transplant. Bone Marrow Transplant. 39:121–5. DOI: 10.1038/sj.bmt.1705549. PMID: 17173054.

8. Gratwohl A, Brand R, McGrath E, van Biezen A, Sureda A, Ljungman P, et al. 2014; Use of the quality management system "JACIE" and outcome after hematopoietic stem cell transplantation. Haematologica. 99:908–15. DOI: 10.3324/haematol.2013.096461. PMID: 24488562. PMCID: PMC4008105.

9. Kamble R, Pant S, Selby GB, Kharfan-Dabaja MA, Sethi S, Kratochvil K, et al. 2005; Microbial contamination of hematopoietic progenitor cell grafts-incidence, clinical outcome, and cost-effectiveness: an analysis of 735 grafts. Transfusion. 45:874–8. DOI: 10.1111/j.1537-2995.2005.04178.x. PMID: 15934984.

10. Lazarus HM, Magalhaes-Silverman M, Fox RM, Creger RJ, Jacobs M. 1991; Contamination during in vitro processing of bone marrow for transplantation: clinical significance. Bone Marrow Transplant. 7:241–6.
11. Watz E, Remberger M, Ringden O, Ljungman P, Sundin M, Mattsson J, et al. 2015; Quality of the hematopoietic stem cell graft affects the clinical outcome of allogeneic stem cell transplantation. Transfusion. 55:2339–50. DOI: 10.1111/trf.13143. PMID: 25968813.

12. Cobo F, Cortés JL, Cabrera C, Nieto A, Concha A. 2007; Microbiological contamination in stem cell cultures. Cell Biol Int. 31:991–5. DOI: 10.1016/j.cellbi.2007.03.010. PMID: 17452110.

13. Wuchter P. Carreras E, Dufour C, editors. 2019. Processing, cryopreserving and controlling the quality of HSCs. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Springer;Cham: p. 127–30.

14. Donmez A, Aydemir S, Arik B, Tunger A, Cilli F, Orman M, et al. 2012; Risk factors for microbial contamination of peripheral blood stem cell products. Transfusion. 52:777–81. DOI: 10.1111/j.1537-2995.2011.03359.x. PMID: 21981571.

15. Clark P, Trickett A, Saffo S, Stark D. 2014; Effects of cryopreservation on microbial-contaminated cord blood. Transfusion. 54:532–40. DOI: 10.1111/trf.12323. PMID: 23808601.

16. Klein MA, Kadidlo D, McCullough J, McKenna DH, Burns LJ. 2006; Microbial contamination of hematopoietic stem cell products: incidence and clinical sequelae. Biol Blood Marrow Transplant. 12:1142–9. DOI: 10.1016/j.bbmt.2006.06.011. PMID: 17085307.

17. Snowden JA, Saccardi R, Orchard K, Ljungman P, Duarte RF, Labopin M, et al. 2020; Benchmarking of survival outcomes following haematopoietic stem cell transplantation: a review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 55:681–94. DOI: 10.1038/s41409-019-0718-7. PMID: 31636397. PMCID: PMC7113189.
18. Kang ES, Lim CS, Han KS, Kim DW. 2020; Hematopoietic stem cell registry report: 2018. Korean J Blood Transfus. 31:109–18. DOI: 10.17945/kjbt.2020.31.2.109.

19. Almeida ID, Schmalfuss T, Röhsig LM, Goldani LZ. 2012; Autologous transplant: microbial contamination of hematopoietic stem cell products. Braz J Infect Dis. 16:345–50. DOI: 10.1016/j.bjid.2012.06.012. PMID: 22846122.

20. Jacobs MR, Good CE, Fox RM, Roman KP, Lazarus HM. 2013; Microbial contamination of hematopoietic progenitor and other regenerative cells used in transplantation and regenerative medicine. Transfusion. 53:2690–6. DOI: 10.1111/trf.12150. PMID: 23461309.

21. Owers KL, James E, Bannister GC. 2004; Source of bacterial shedding in laminar flow theatres. J Hosp Infect. 58:230–2. DOI: 10.1016/j.jhin.2004.06.028. PMID: 15501339.

22. Anatoliotaki M, Mantadakis E, Galanakis E, Samonis G. 2003; Rhodotorula species fungemia: a threat to the immunocompromised host. Clin Lab. 49:49–55.
23. Goenaga Sánchez MA, Alberdi F, Carrera JA, Millet Sampedro M, Garde Orbaiz C. 2003; [Leuconostoc spp bacteremia in a patient with intestinal pseudoobstruction]. An Med Interna. 20:53–4. DOI: 10.4321/S0212-71992003000100020. PMID: 12666318.
24. Prince HM, Page SR, Keating A, Saragosa RF, Vukovic NM, Imrie KR, et al. 1995; Microbial contamination of harvested bone marrow and peripheral blood. Bone Marrow Transplant. 15:87–91.
25. Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, et al. 2015; Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 373:1220–9. DOI: 10.1056/NEJMoa1500964. PMID: 26398070.

26. O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. 2002; Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 51:1–29.
27. Shi Y, Yang N, Zhang L, Zhang M, Pei HH, Wang H. 2019; Chlorhexidine disinfectant can reduce the risk of central venous catheter infection compared with povidone: a meta-analysis. Am J Infect Control. 47:1255–62. DOI: 10.1016/j.ajic.2019.02.024. PMID: 30948152.

28. Namdaroğlu S, Tekgündüz E, Bozdağ SC, Durgun G, Sarıca A, Demiriz IŞ, et al. 2013; Microbial contamination of hematopoietic progenitor cell products. Transfus Apher Sci. 48:403–6. DOI: 10.1016/j.transci.2013.04.026. PMID: 23664302.

29. Patah PA, Parmar S, McMannis J, Sadeghi T, Karandish S, Rondon G, et al. 2007; Microbial contamination of hematopoietic progenitor cell products: clinical outcome. Bone Marrow Transplant. 40:365–8. DOI: 10.1038/sj.bmt.1705731. PMID: 17572714.

30. Damonti L, Buetti N, Droz S, Bacher U, Pabst T, Taleghani BM, et al. 2021; Prevalence and significance of bacterial contamination of autologous stem cell products. J Hosp Infect. 114:175–9. DOI: 10.1016/j.jhin.2021.04.006. PMID: 33864895.

31. Dal MS, Tekgündüz E, Çakar MK, Kaya AH, Namdaroğu S, Batgi H, et al. 2016; Does microbial contamination influence the success of the hematopoietic cell transplantation outcomes? Transfus Apher Sci. 55:125–8. DOI: 10.1016/j.transci.2016.05.001. PMID: 27184293.

32. Kozlowska-Skrzypczak M, Bembnista E, Kubiak A, Matuszak P, Schneider A, Komarnicki M. 2014; Microbial contamination of peripheral blood and bone marrow hematopoietic cell products and environmental contamination in a stem cell bank: a single-center report. Transplant Proc. 46:2873–6. DOI: 10.1016/j.transproceed.2014.09.002. PMID: 25380939.

33. Corrias MV, Haupt R, Carlini B, Parodi S, Rivabella L, Garaventa A, et al. 2006; Peripheral blood stem cell tumor cell contamination and survival of neuroblastoma patients. Clin Cancer Res. 12:5680–5. DOI: 10.1158/1078-0432.CCR-06-0740. PMID: 17020970.

Fig. 1
Annual numbers of hematopoietic stem cell products collected and the proportion of culture-positive products between January 1996 and May 2021.
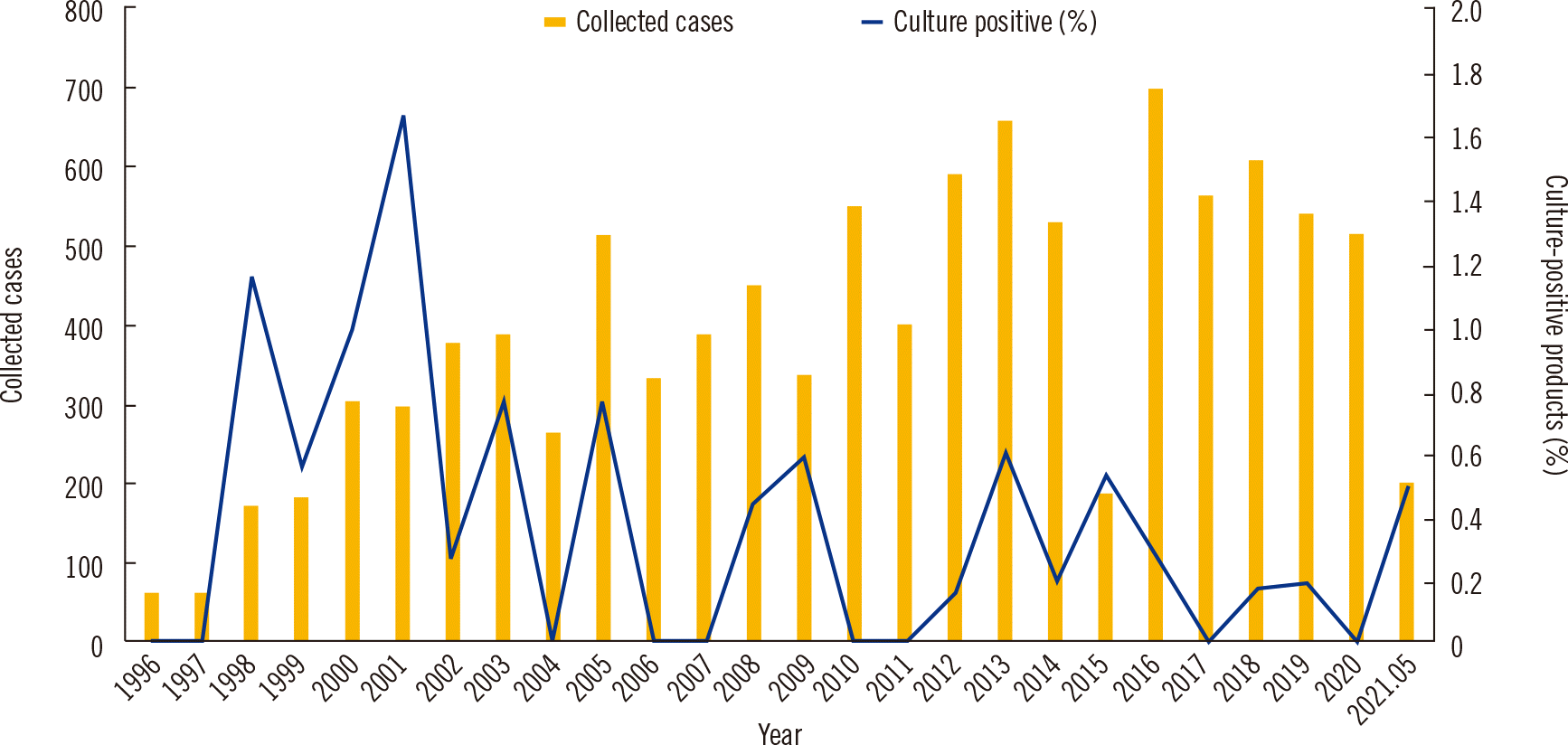
Table 1
Changes in the reorganized CTL
Table 2
Microbial culture results for HSC products and other samples and potential sources/steps of contamination
Table 3
Transplanted culture-positive HSC products and patient outcomes
| Case number | Diagnosis | Sample type | Microbial culture | Source/step of contamination | Prophylactic antibiotics | Evidence of infection* | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Neuroblastoma, stage IV | Auto-PBSC | Pseudomonas aeruginosa | Collection/processing | Teicoplanin, ticarcillin/clavulanic acid, gentamicin | Not apparent | Engrafted |
| 2 | Severe aplastic anemia | Allo-BM | Corynebacterium jeikeium | Culture preparation | Acyclovir, cefepime, teicoplanin, micafungin | Not apparent | Engrafted |
| 3 | Acute myeloid leukemia | Allo-PBSC | Leuconostoc pseudomesenteroides | Culture preparation | Acyclovir, cefepime, micafungin | Not apparent | Engrafted |
| 4 | Acute myeloid leukemia | Allo-PBSC | Streptococcus salivarius | Culture preparation | Acyclovir, voriconazole | Not apparent | Engrafted |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download