Abstract
Background
The currently recommended pre-transfusion testing techniques for patients with autoantibodies are complex, time-consuming, and labor-intensive. Therefore, although the red blood cell (RBC) selection method using crossmatched RBC agglutination reaction grades (i.e., the “least incompatible” transfusion) is discouraged, many institutions still use it. We aimed to evaluate the effectiveness of this method combined with Rh subgroup phenotyping.
Methods
We retrospectively investigated RBC transfusions from January 2019 to December 2021 in patients presenting as auto-control-positive via antibody identification (auto-control (+) group), where Rh subgroup phenotype-matched RBCs were selected based on the agglutination reaction grades of crossmatched units. For each study patient, an auto-control-negative patient was matched based on age, sex, department, and pre-transfusion Hb levels (auto-control (−) group). The mean Hb change per unit, transfusion-associated symptom/sign reports, and agglutination reaction grades upon crossmatching were analyzed.
Results
In the auto-control (+) group, the Hb change per unit among different agglutination reaction grades of transfused RBCs and among different relative grades of transfused RBCs and crossmatching auto-controls was not significantly different (P=0.392 and P=0.132, respectively). No significant difference was observed in Hb changes and transfusion-associated symptom/sign occurrence between the auto-control (+) and auto-control (−) groups (P=0.121 and P=0.822, respectively). In addition, no definite evidence of hemolysis in the auto-control (+) group was observed in the medical record review.
Pre-transfusion testing aims to achieve an adequate transfusion effect without harming the patient by providing an optimal blood product. Many serological approaches, such as ABO/Rh typing, antibody screening or identification, and crossmatching techniques, are routinely performed in blood banks, enabling the accurate identification of blood types and unexpected antibodies. Although these procedures have succeeded in matching compatible blood in most cases, some challenges remain such as for patients with autoantibodies [1, 2]. In such cases, the patient’s serum can react with normal red blood cells (RBCs), masking the presence of alloantibodies that could cause hemolytic transfusion reactions [3]. There are several techniques for detecting and identifying alloantibodies in the presence of underlying autoantibodies, including serum dilution and adsorption [3]. However, there are no evidence-based protocols to guide testing and RBC selection [4–6].
The “least incompatible” transfusion method is a simple method for selecting blood units based on agglutination reaction grades of crossmatched RBCs. However, no high-level evidence supports the effectiveness and safety of choosing the “least incompatible” unit. Although its use is discouraged [7], blood banks that cannot afford additional procedures such as dilution or adsorption, which are time-consuming and labor-intensive, still adopt the “least incompatible” transfusion method. Studies have reported the effect of “least compatible” transfusion on patients with autoimmune hemolytic anemia (AIHA) [8, 9]. Park, et al. [8] evaluated the increase in Hb levels and changes in total bilirubin and lactate dehydrogenase levels in patients with AIHA and reported that “least incompatible” RBC transfusion was effective for patients with AIHA and does not increase the hemolysis risk. In a retrospective study based on 450 hospitalized patients with AIHA, Chen, et al. [9] showed that the “least incompatible” blood did not adversely affect the transfusion efficiency. However, these previous studies did not include an evaluation based on agglutination reaction grades of the transfused RBCs or the relative strength of agglutination reaction grades between the transfused RBCs and the crossmatched auto-control. Therefore, we aimed to evaluate the effectiveness of RBC transfusion using crossmatch agglutination reaction grades (combined with Rh subgroup phenotyping) by analyzing agglutination reaction grades, transfusion-associated symptoms/signs, and the change in Hb levels based on a three-year experience.
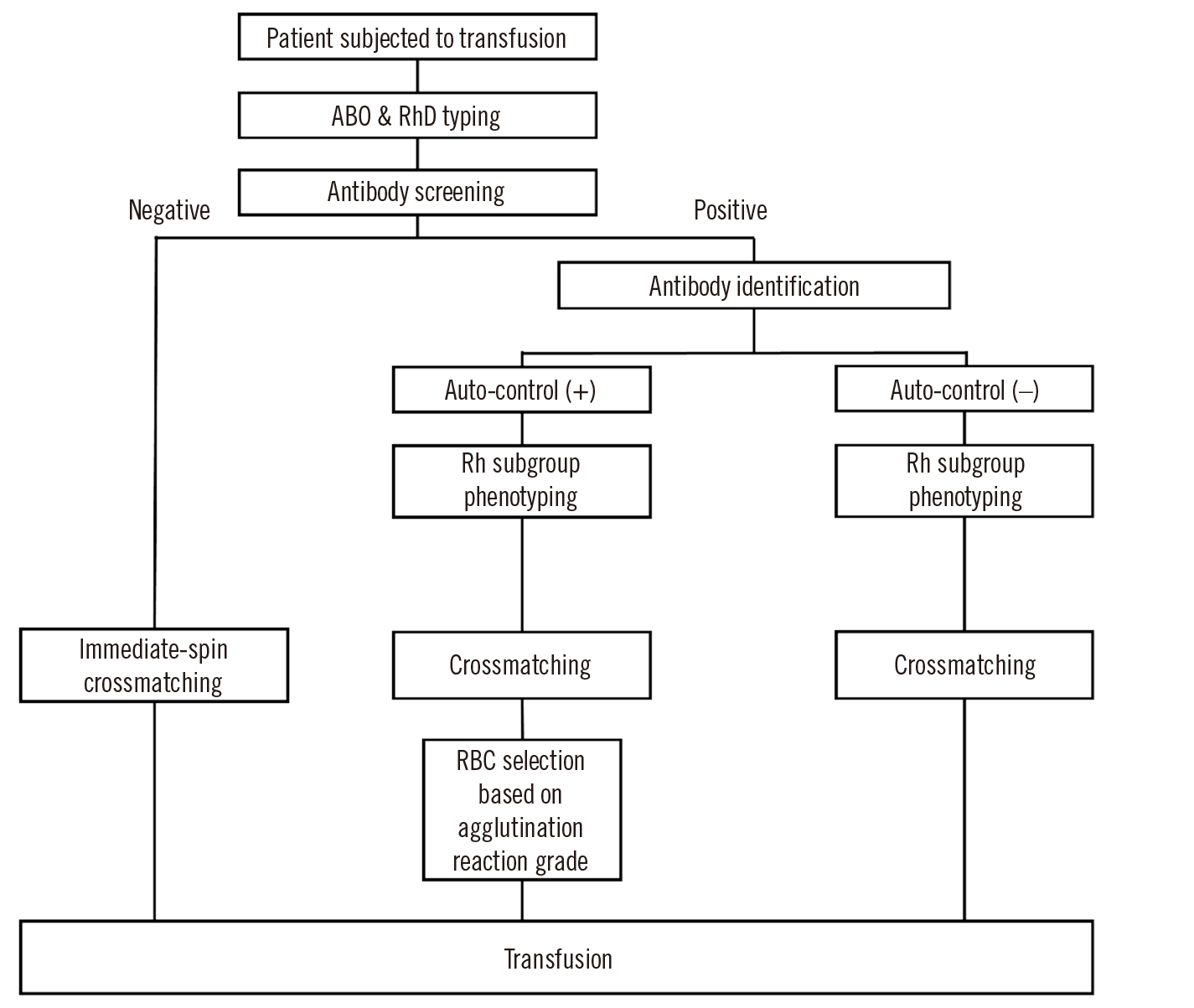
ABO/RhD typing and antibody screening were performed for all patients subjected to transfusion (Fig. 1). ID-DiaCell I-II-Dia (DiaMed, Murten, Switzerland) and LISS/Coombs ID-card (DiaMed) were used for antibody screening. The type-and-screen method was applied for antibody screening-negative results. For antibody screening-positive results, an antibody ID test was performed using ID-Dia Panel 1-11 (DiaMed) with both an LISS/Coombs ID-card and NaCl, Enzyme Test and Cold Agglutinins ID-card (DiaMed) at 37°C. Additional tests were performed at 4°C when cold-reacting antibodies were suspected. Subsequently, patients were subjected to Rh subgroup phenotyping, including C, c, E, e, and K antigens, using the Ortho BioVue System Rh/K cassette (Ortho-Clinical Diagnostics, Pencoed, UK). Trained laboratory technicians interpreted the results of the Rh subgroup phenotyping to determine agglutination reaction grades. Results were considered positive when the agglutination reaction grade of the antigen testing column was stronger than that of the auto-control, negative when weaker, and uninterpretable when equal. Following Rh subgroup phenotyping, crossmatching was performed using an LISS/Coombs ID-card (DiaMed) at 37°C. Agglutination reactions were interpreted and graded as 0, ±, 1+, 2+, 3+, and 4+.
This study was approved by the Pusan National University Hospital Institutional Review Board (2209-010-118) that also waived the need to obtain consent for the collection, testing, and publication of retrospectively obtained and anonymized data for this non-interventional study.
Patients with auto-control-positive results in the antibody ID test (i.e., the auto-control (+) group) were subjected to RBC selection based on the crossmatching agglutination reaction grades. The primary goal was to select the ABO/RhD-compatible and Rh subgroup phenotype-matched RBC units with the lowest agglutination reaction grade among RBC units and a lower agglutination reaction grade than that of the crossmatching auto-control (XM auto-control). When the available RBC units did not meet the criteria, RBCs demonstrating agglutination reaction grades equal to those of the auto-control were selected, except for cases with the auto-control presenting a grade of 4+. When RBCs presenting lower or equal agglutination reaction grades compared with those of the auto-control were unavailable or when all RBCs demonstrated a grade of 4+, the RBCs were released from clinical consideration after consultation with the patient’s physician.
RBC transfusions performed from January 2019 to December 2021 were retrospectively investigated using electronic medical records, including those of the auto-control (+) group. For each patient in the auto-control (+) group, an auto-control-negative patient was matched based on age, sex, department, and pre-transfusion Hb levels, which was referred to as the auto-control (−) group. The numbers of RBC transfusions, transfused RBC units, Rh subgroup phenotypes, and pre- and post-transfusion Hb levels were analyzed. Agglutination reaction grades of the auto-control and transfused RBCs in crossmatching were retrospectively investigated based on documented paper records.
For each RBC transfusion, healthcare providers observed and recorded post-transfusion symptoms and signs of the transfused patient in a standardized report form—the transfusion-associated symptom/sign report. The report consisted of nine groups of symptoms and signs: “no symptom,” “fever/chills,” “urticaria/pruritus/rash,” “high/low blood pressure,” “chest discomfort/dyspnea,” headache/dizziness/nausea/vomiting,” “hemorrhage/hematuria/oliguria,” “pain,” and “others.”
The mean change in Hb per unit was analyzed to evaluate the effect of RBC transfusion. The pre-transfusion Hb level was defined as the latest Hb level from 0 to 24 hours before transfusion. The post-transfusion Hb level was defined as the earliest Hb level from 4 to 24 hours after transfusion. The change between pre- and post-transfusion Hb levels was divided by the number of transfused RBC units and defined as the mean change in Hb per unit.
The medical records were reviewed for auto-control (+) and auto-control (−) group patients presenting with signs based on the transfusion-associated symptom/sign reports. In addition, patients who demonstrated a significant decrease in Hb, defined as the box plot extreme outliers (75th percentile+3×interquartile range or 25th percentile −3×interquartile range), were subjected to medical record review to assess hemolytic transfusion reactions. Medical record review included complete blood count results; peripheral blood smear results; urinalyses; direct and indirect bilirubin, lactate dehydrogenase, and haptoglobin levels; and direct antigen test results.
Primary analysis compared the auto-control (+) and auto-control (−) groups. Normality tests were performed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Continuous variables are described as means with SDs, and categorical variables are described as counts and percentages. The Kruskal–Wallis test was used to compare the change in Hb between different agglutination reaction grades of the transfused RBCs and to analyze Hb changes among different relative agglutination reaction grades between the auto-control and transfused RBCs. The chi-square test was used to analyze the baseline characteristics of the auto-control (+) and (−) groups and the occurrence of transfusion-associated symptom/signs between the two groups. A two-sample t-test was used to analyze the Hb change per unit between the auto-control (+) and (−) groups. Statistical significance was set at P<0.05. All statistical analyses were performed and box plots were generated using SPSS version 22 for Windows (IBM Corp., Armonk, NY, USA).
In total, 57,250 RBC units were transfused during the three-year study period. RBC transfusion in 55 patients in the auto-control (+) group accounted for 0.7% (N=388) of the total transfusions. Among 55 patients, 49 (89.1%) were subjected to Rh subgroup phenotyping, demonstrating the following results: 23 (41.8%), 8 (14.6%), 5 (9.1%), and 3 (5.4%) patients showed the CCee, CcEe, ccEE, and Ccee phenotypes, respectively. The results of 10 patients (18.2%) were not interpretable because all tested columns had grades of 4+. Eleven of 388 (2.8%) units were involved in transfusion reactions as follows: 10 units (six patients) associated with fever/chills and 1 unit (one patient) with urticaria/pruritus/rash.
In the auto-control (+) group, the agglutination reaction grades of the auto-controls in crossmatching for 246 RBC transfusions were distributed as follows: 20 (8.1%) negative, 30 (12.2%) ±, 46 (18.7%) 1+, 89 (36.2%) 2+, 49 (19.9%) 3+, and 12 (4.9%) 4+ (data not shown). Table 1 presents the results of Hb changes per unit among different agglutination reaction grades of the transfused RBCs in crossmatching. There were no significant differences in Hb changes among the different agglutination reaction grades of the transfused RBCs (P=0.418). Table 1 also presents Hb changes per unit in groups classified by relative agglutination reaction grades between transfused RBCs and auto-controls in crossmatching (XM auto-control). The difference in Hb changes was not significant between the groups (P=0.165).
The baseline characteristics of the auto-control (+) and auto-control (−) groups are shown in Table 2. The analyzed variables showed no significant differences between the groups; the changes in Hb levels (Fig. 2, P=0.121) and transfusion-associated symptom/sign occurrence [11/388 (2.8%) in auto-control (+) vs. 9/385 (2.3%) in auto-control (-), P=0.822] also did not differ significantly. Nine RBC units of the auto-control (−) group were associated with one or more transfusion-associated symptom/signs: seven units (five patients) with fever/chills, two with high/low blood pressure (two patients), and two (one patient) with urticarial/rash (data not shown).
None of the transfused RBCs from seven patients of the auto-control (+) group presenting with transfusion-associated symptoms/signs showed agglutination in crossmatching (Table 3). In addition, none of these cases showed definite evidence of hemolytic events after transfusion. However, two cases of significantly decreased Hb levels after transfusion were associated with hemolytic characteristics, both of which were associated with suspicions of Evan’s syndrome before their first transfusion (Table 3). None of the auto-control (−) group patients with transfusion-associated symptoms/signs or with significantly decreased Hb levels had indications of hemolysis or clinical situations that could influence Hb levels.
The Hb change among agglutination reaction grades of transfused RBCs in crossmatching did not present a significant difference. Similarly, analysis by groups that were classified based on relative agglutination reaction grades between the transfused units and crossmatching auto-control (XM auto-control) showed no significant difference in the Hb change. However, this analysis was limited by the small sample size of some groups (“agglutination reaction grade 4+”, “transfused RBCs >XM auto-control”, and “XM auto-control=4+ and transfused RBCs=4+” in Table 1). Nevertheless, these groups showed a lower average mean Hb change per unit, indicating that caution is required when the agglutination reaction grade of the transfused RBCs is higher than that of the XM auto-control or when the comparison is unavailable (such as for “XM auto-control=4+ and transfused RBCs=4+” in Table 1). Further studies are required to understand when the degree of agglutination is predictive of the transfusion outcome.
An unexpected result was that the mean Hb change per unit for “XM auto-control=0 and transfused RBCs=0” was relatively lower than that for “XM auto-control<transfused RBC” and “XM auto-control=transfused RBCs.” Although the group “XM auto-control=0 and transfused RBCs=0” is the safest choice and is expected to show a higher increase in Hb, patient conditions (such as bleeding or underlying comorbidities) might have been influenced transfusion outcomes. Nevertheless, since there was no significant difference in the Hb change between the safest-known RBCs and RBCs from “XM auto-control <transfused RBCs” or “XM auto-control=transfused RBCs,” the method of interest seems to be useful in providing effective transfusion.
The analysis between the auto-control (+) and (−) groups demonstrated no significant difference in the Hb change and transfusion-associated symptom/sign occurrence. In addition, a medical record review showed that all patients presenting with transfusion-associated symptoms/signs demonstrated no agglutination reactions in transfused RBCs, and no evidence of hemolysis was noted. Therefore, together with the medical record review, the method of interest was considered to be effective without inducing symptomatic hemolytic events.
Autoantibodies with mimicking specificity could interfere with the issuance of compatible blood. For example, blood selection for a patient with the Ce phenotype in the presence of autoantibodies with mimicking specificity for RhC can be challenging. When transfusion is considered necessary, choosing antigen-negative RBCs is generally considered prudent, despite the possible risk of antithetical antigen exposure [10]. However, Issitt, et al. [11] speculated that providing antigen-negative RBCs for patients having mimicking autoantibodies is unnecessary. Further, Jang, et al. [12] reported that “least compatible” RBC transfusions administered to five patients with mimicking autoantibodies did not induce severe acute or delayed hemolytic transfusion reactions. We did not perform an additional investigation on the mimicking specificity of the autoantibodies. However, clinically significant mimicking autoantibodies will be detected in the crossmatching stage, and if the degree of agglutination is below that of the auto-control, it will be considered acceptable according to the method of interest. Given that mimicking autoantibodies are estimated to be present in 12.0%–26.8% of patients with warm reactive autoantibodies [11–13], the results of our study show that the method of interest can be effective for patients with autoantibodies, including those with mimicking autoantibodies.
This study has several limitations, including those mentioned above. The method adopted in this study was performed in combination with preceding pre-transfusion testing; thus, the evaluation of the utility of this approach as an independent method is limited. However, it is known that pre-transfusion testing performed before crossmatching in the protocol of this study was insufficient to provide a measure of safety, and the addition of the method of interest showed favorable results. Second, this study did not investigate the long-term outcomes of the transfused patients. Lastly, clinical situations that can influence Hb levels were not investigated for all patients. However, patients with suspicion of having hemolysis, such as those presenting with signs after transfusion or those who demonstrated a significant decrease in Hb levels, were analyzed through medical record reviews. In addition, the vast majority of clinical situations results in a decrease in Hb levels, such as with bleeding, whereas only few rare events might increase Hb levels. Therefore, although the effect of transfusion is compromised and could have been underestimated, transfusion using the method of interest was effective.
In conclusion, combined with Rh subgroup phenotyping, selecting the RBC unit with the lowest agglutination reaction grade among crossmatched RBCs did not adversely affect the transfusion efficiency. Therefore, we believe that this method will be helpful in institutions where complex techniques are unavailable.
Notes
REFERENCES
1. Garratty G, Petz LD. 2002; Approaches to selecting blood for transfusion to patients with autoimmune hemolytic anemia. Transfusion. 42:1390–2. DOI: 10.1046/j.1537-2995.2002.00284.x. PMID: 12421209.

2. Salama A, Berghöfer H, Mueller-Eckhardt C. 1992; Red blood cell transfusion in warm-type autoimmune haemolytic anaemia. Lancet. 340:1515–7. DOI: 10.1016/0140-6736(92)92766-9. PMID: 1361607.

3. Petz LD. 2004; A physician's guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 124:712–6. DOI: 10.1111/j.1365-2141.2004.04841.x. PMID: 15009058.

4. Ziman A, Cohn C, Carey PM, Dunbar NM, Fung MK, Greinacher A, et al. 2017; Warm-reactive (immunoglobulin G) autoantibodies and laboratory testing best practices: review of the literature and survey of current practice. Transfusion. 57:463–77. DOI: 10.1111/trf.13903. PMID: 27917465.

5. Reardon JE, Marques MB. 2006; Laboratory evaluation and transfusion support of patients with autoimmune hemolytic anemia. Am J Clin Pathol. 125:S71–7. DOI: 10.1309/ABP0B54GQL74L3KW. PMID: 16830958.

6. Leger RM, Garratty G. 1999; Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 39:11–6. DOI: 10.1046/j.1537-2995.1999.39199116889.x. PMID: 9920161.

7. Petz LD. 2003; "Least incompatible" units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 43:1503–7. DOI: 10.1046/j.1537-2995.2003.00583.x. PMID: 14617306.

8. Park SH, Choe WH, Kwon SW. 2015; Red blood cell transfusion in patients with autoantibodies: is it effective and safe without increasing hemolysis risk? Ann Lab Med. 35:436–44. DOI: 10.3343/alm.2015.35.4.436. PMID: 26131416. PMCID: PMC4446583.

9. Chen C, Wang L, Han B, Qin L, Ying B. 2020; Autoimmune hemolytic anemia in hospitalized patients: 450 patients and their red blood cell transfusions. Medicine. 99:e18739. DOI: 10.1097/MD.0000000000018739. PMID: 31914091. PMCID: PMC6959959.
10. Dwyre DM, Clapper A, Heintz M, Elbert C, Strauss RG. 2004; A red blood cell autoantibody with mimicking anti-E specificity. Transfusion. 44:1287–92. DOI: 10.1111/j.0041-1132.2004.04112.x. PMID: 15318850.

11. Issitt PD, Combs MR, Bumgarner DJ, Allen J, Kirkland A, Melroy-Carawan H. 1996; Studies of antibodies in the sera of patients who have made red cell autoantibodies. Transfusion. 36:481–6. DOI: 10.1046/j.1537-2995.1996.36696269503.x. PMID: 8669076.

12. Jang MJ, Cho D, Park KU, Yazer MH, Shin MG, Shin JH, et al. 2013; Autoantibodies with mimicking specificity detected by the dilution technique in patients with warm autoantibodies. Ann Lab Med. 33:343–8. DOI: 10.3343/alm.2013.33.5.343. PMID: 24003424. PMCID: PMC3756238.

13. Wheeler CA, Calhoun L, Blackall DP. 2004; Warm reactive autoantibodies: clinical and serologic correlations. Am J Clin Pathol. 122:680–5. DOI: 10.1309/CJAW6N8J6H0HR2WM. PMID: 15491963.
Fig. 2
Box-and-whisker plot of Hb change per unit between auto-control (+) and auto-control (–) groups. The top of the box represents the 75th percentile, the middle of the box represents the 50th percentile, and the bottom represents the 25th percentile. The whiskers represent the maximum and minimum values, excluding outliers. Open circles indicate outliers (75th percentile+1.5×interquartile range or 25th percentile−1.5×interquartile range) and asterisks indicate extreme outliers (75th percentile+3×interquartile range or 25th percentile−3×interquartile range), which were subjected to medical record review.

Table 1
Evaluation of Hb change per unit in the auto-control (+) group
Table 2
Baseline characteristics of auto-control (+) and auto-control (−) groups
Table 3
Medical record review of patients in the auto-control (+) group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download