Abstract
Background
The response to vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) varies depending on comorbidities. This study evaluated the clinical and immunological factors affecting the humoral response of patients with end-stage renal disease (ESRD) to the BNT162b2 vaccine.
Methods
Humoral immunity was evaluated in 54 ESRD patients using serum levels of anti-receptor-binding domain (RBD) and neutralizing antibodies (NAbs), measured by a chemiluminescent immunoassay 30 (T1), 60 (T2), and 120 (T3) days after the second vaccine dose. The results were correlated to baseline patient T- and B-lymphocyte subpopulations determined by flow cytometry.
Results
The proportion of seroconverted patients based on the NAb titer decreased from 83.3% at Τ1 to 53.7% at Τ3. Age was negatively correlated to the NAb titer at T1 and Τ2. Patients receiving hemodiafiltration had higher NAb titers at T3. Diabetes was associated with a lower response rate at T3. Univariate analysis revealed a positive correlation between the naïve CD4 T-lymphocyte population and RBD titer at T1 and the NAb titer at T3, with no association observed with naïve CD8 T lymphocytes. NAb titers at T3 were significantly correlated with late-differentiated CD4 T lymphocytes and terminally differentiated effector memory cells re-expressing CD45RA (TEMRA) CD8 T lymphocytes. RBD levels were positively correlated with naïve and memory B-lymphocyte counts at T3.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), resulted in a loss of more than 28 million years of life in 2020 alone [1]. One of the major factors responsible for increased morbidity and mortality associated with COVID-19 is immune system deficiency, which is often related to conditions such as older age, chronic disease, or immunosuppressive treatment. Moreover, individuals in these categories are more likely to demonstrate inadequate responses to vaccination [2].
The SARS-CoV-2 virion consists of four structural proteins, of which the spike (S) protein plays the most important role in viral infection [3]. The high immunogenicity of this protein has led to the development of vaccines containing S protein-encoding mRNA encapsulated in lipid nanoparticles. Two mRNA vaccines were approved in Greece: BNT162b2 (Pfizer-BioNTech, Pfizer Inc., New York, NY, USA) and mRNA-1273 (Moderna, Cambridge, MA, USA). Both vaccines have been used in patients with end-stage renal disease (ESRD). The effect of ESRD on the immune system resembles that of advanced age [4]. Acquired immunity is greatly affected with prominent phenotypic and functional alterations of lymphocytes, such as elimination of naïve subsets and antigen repertoire restriction, proliferation of highly differentiated lymphocyte populations, and increased expression of proinflammatory mediators [4].
An impaired immune response to vaccination is of major clinical importance, and immune disorders exclusively found in ESRD are expected to have a determinant role. However, investigations of the impact of immune disorders on vaccination efficacy are limited. In this study, we evaluated the humoral response to mRNA vaccines against COVID-19 among ESRD patients and assessed the effect of phenotypic alterations of lymphocytes on antibody production.
This prospective observational study was designed to evaluate the specific effect of certain lymphocyte phenotypic alterations in response to mRNA vaccination against COVID-19 in patients with ESRD undergoing maintenance hemodialysis three times a week. For this purpose, we recruited 54 patients with ESRD who had been undergoing dialysis treatment for at least 1 year in the dialysis unit of Hippokration Hospital of Thessaloniki, Greece. All patients received their first dose of an mRNA vaccine (BNT162b2, Pfizer-BioNTech) between April 1 and April 20, 2021 (T0), followed by the second dose 3 weeks later. After the second dose, patients were followed up for 4 months, and blood samples were collected on the 30th (T1), 60th (T2), and 120th (T3) day. Blood samples were centrifuged at 500 ×g for 10 minutes, and sera were collected and stored at -60°C until processing.
T- and B-lymphocyte subsets were assessed by flow cytometric analysis performed on the day of the first dose of vaccination (T0). The gating strategy is illustrated in Supplemental Data Fig. S1. The humoral response to vaccination was evaluated at T1, T2, and T3 based on serum levels of antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 S1 protein and the neutralizing antibody (NAb) titer, and the response level was correlated to the specific immune phenotypes estimated by flow cytometry at T0.
Flow cytometry
Flow cytometry was performed on peripheral blood drawn at the beginning of a mid-week dialysis session in K2EDTA tubes. The blood was immediately transferred to the laboratory, processed within 12 hours, and stored at 4°C until staining. Blood samples were stained with conjugated antibodies, including anti-CD45 PC7 IM3548U (Beckman Coulter, Brea, CA, USA), anti-CD3 fluorescein isothiocyanate (FITC) UCHT1 (Beckman Coulter), anti-CD3 phycoerythrin (PE) UCHT1 (Beckman Coulter), anti-CD4 Pacific blue EM4 (EXBIO, Praha SA, Vestec, Czech Republic), anti-CD8 PC5 (IM3548U Beckman Coulter), anti-CD45RA allophycocyanin (APC) MEM-56 (EXBIO, Praha SA), anti-CCR7 PE 4B12 (EXBIO, Praha SA), anti-CD28 CD28.2 PE-EF610 (Thermo Scientific LSG, Waltham, MA, USA), anti-CD31 APC MEM-05 (EXBIO, Praha SA), anti-CD57 FITC (EXBIO, Praha SA), anti-CD19 PC5 J3-119 (Beckman Coulter), anti-CD27 PE-DyLight 594 (EXBIO, Praha SA), and anti-IgD IA6-2 (Thermo Scientific LSG). B lymphocytes were washed twice with phosphate-buffered saline before flow cytometry to remove soluble IgD. CD4+, CD8+, and B-lymphocyte subsets were determined using a cell counter (Navios Flow Cytometer, Beckman Coulter) according to the manufacturer’s recommendations and determined by the expression of surface markers estimated at time T0 as follows: CD4+ and CD8+ lymphocytes: CD45RA+CCR7+ (naïve), CD45RA+CD31+ (recent thymic emigrants [RTEs]), CD45RA+CCR7– (terminally differentiated effector memory cells re-expressing CD45RA [TEMRA]), and CD28+CD57– and CD28–CD57+ (late-differentiated [LD]) [4]; B lymphocytes: CD19+IgD+CD27- (naïve B cells) and CD19+CD27+ (memory B cells) [5].
In all patients, we determined SARS-CoV-2–specific antibody reactions using the MAGLUMI SARS-CoV-2-S-RBD IgG kit (Shenzhen New Industries Biomedical Engineering Co., Ltd. [SNIBE], Shenzhen, China), which is a fully automated indirect chemiluminescence immunoassay that detects antibodies directly against the RBD of the S1 protein. According to the manufacturer’s instruction, 10 μL of patient sera, buffer, and magnetic microbeads coated with S-RBD recombinant antigen were mixed thoroughly and incubated. After precipitation in a magnetic field, the beads were washed, and amino-butyl-ethyl-isoluminol (ABEI)-labeled anti-human IgG antibody was added and incubated to form complexes. The light signal of the chemiluminescent reaction was measured using a photomultiplier (MAGLUMI 800, SNIBE) in relative light units (RLUs), based on which the analyzer automatically calculated the IgG SARS-CoV-2 S-RBD level using a calibration curve. Sera with an IgG level >100 AU/mL were diluted, and the antibody titer was calculated automatically using the analyzer software. Antibody levels above 1.0 AU/mL were considered positive.
We also analyzed NAb levels against SARS-CoV-2 using the MAGLUMI SARS-CoV-2 Neutralizing Antibody assay (SNIBE). The SARS-CoV-2 NAb present in the sample competes with the angiotensin-converting enzyme 2 (ACE2) antigen immobilized on magnetic microbeads for binding to the recombinant SARS-CoV-2 S-RBD antigen labeled with ABEI. The light signal of the chemiluminescent reaction was measured by a photomultiplier in RLU, which is inversely proportional to the level of the SARS-CoV-2 NAb present in the sample. Sera with IgG levels >30 μg/mL were diluted, and the antibody titer was calculated automatically using the analyzer software. NAb levels above 0.3 μg/mL were considered positive. Patients with NAb titers above the cut-off level were regarded as responders (seroconversion).
The study was conducted at the Department of Nephrology, Medical School, Aristotle University of Thessaloniki, in collaboration with the National Peripheral Histocompatibility Center, Hippokration Hospital, Thessaloniki, Greece. All patients were of European Caucasian ethnicity. The eligibility criteria were aged 20-80 years and receiving an adequate dialysis dosage (Kt/V >1.2) for at least 1 year, achieved either by hemodialysis (HD) or hemodiafiltration (HDF) thrice a week. Patients with concomitant systemic diseases, malignancies, hematological disorders; recent infection (<3 months of recovery); recent vaccination against influenza, hepatitis B, or pneumococcus (<2 months); or concomitant steroid or immunosuppressive treatment for the last 3 months were excluded from the study. Patients with a history of COVID-19 were also excluded from the study, regardless of the time since recovery.
All patients signed an informed consent form before enrollment. The study was approved by the Institutional Review Board of the Medical School of the Aristotle University of Thessaloniki (Ref No. 2.273/15-12-2020) and was conducted in accordance with the Declaration of Helsinki.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 25 for Windows (IBM, Armonk, NY, USA). Continuous variables were examined for normality and are expressed as mean±SD for normally distributed variables or as median (25th-75th percentile) for non-normally distributed variables. Differences between two unrelated variables were estimated using the Mann–Whitney U-test, whereas differences between two related variables were estimated using Wilcoxon’s test. Analysis of covariance (ANCOVA) was used to evaluate the effect of age on antibody titers in comparisons between subgroups. The chi-square test was used to compare differences between categorical variables. Spearman’s rank correlation coefficient was used to estimate the correlation between age and antibody titers. For the correlation to be considered significant, the R value should be greater than the critical value for the corresponding degrees of freedom. Univariate and multivariate linear regression analyses were performed to estimate independent factors contributing to antibody titers (continuous variables). In regression models, the R2 value was used to determine the percentage of variance in the dependent variable explained by the independent variables. Moreover, beta coefficients (BCs) were estimated to express the expected change in the dependent variable per unit change of the independent variable, whereas partial coefficients (PCs) in multiple regression models were estimated to express the same change, assuming that all other predictor variables are held constant. A P-value<0.05 was considered to indicate statistical significance. The Bonferroni correction was used for multiple comparisons.
Patient demographic characteristics are shown in Table 1. The mean (±SD) patient age was 60 (±13) years. The male/female ratio was 34/20. All patients were undergoing a maintenance hemodialysis program three times per week at our center. All patients had been on dialysis for at least 1 year before inclusion in the study, with a mean dialysis history of 93±66 months. HDF was prescribed to 44.4% of patients. Dates of vaccination of each patient are shown in Supplemental Data Table S1.
We estimated the humoral response of patients on days 30 (T1), 60 (T2), and 120 (T3) after the second dose of vaccination by measuring serum RBD and NAb levels. Nine patients (16.7%) did not reach the cut-off level of 0.3 µg/mL for NAb titers at T1 and were thus regarded as non-responders. This proportion increased to 13/54 (24.1%) at T2 and to 25/54 (46.3%) at T3. Similarly, 2/54 (3.7%), 4/54 (7.4%), and 7/54 (13%) patients could not reach the cut-off RBD level (1.0 AU/mL) at T1, T2, and T3, respectively. The median RBD and NAb titers for all patients dropped significantly from T1 to T3 (RBD: 81.3 [36.6-192.1], 66.6 [24.1-86.6], 48 [11.2-80] AU/mL, P<0.001; NAb: 1.27 [0.5-2.3], 0.93 [0.37-1.84], 0.46 [0.17-1.2] µg/mL, P<0.001, respectively) (Fig. 1).
RBD levels were significantly negatively correlated with age (R=-0.426, P=0.003) only at T1, but not at T2 and T3. Age also had a negative impact on NAb titers at T1 (R=-0.334, P=0.02) and T2 (R=-0.344, P=0.02). At T3, although there was no significant correlation of NAb titers with age, non-responders were significantly older than responders, aged 63 (55.5-72.5) years vs. 55 (48-65) years, respectively (P=0.04). No correlation was found between sex or dialysis history and RBD or NAb levels at any time point.
To define clinical factors that possibly affect the humoral response to vaccination, we subsequently performed a subgroup analysis based on the presence of diabetes mellitus (DM), dialysis modality (HD or HDF), and history of immunosuppression treatment.
The presence of DM appeared to play an important role in the development of humoral responses. Patients with DM (N=11) had significantly lower levels of both RBD and NAb levels at T1 compared to patients without DM (non-DM) (58.6 [11.4-81.7] vs. 98.2 [50-206.9] AU/mL, P=0.05 and 0.53 [0.42-0.71] vs. 1.43 [0.64-2.56] µg/mL, P=0.02, respectively). This difference was retained at T2, despite a reduction in antibody titers in both groups (Fig. 2). ANCOVA for age and sex between the two groups showed that neither factor had an impact on antibody titers between DM and non-DM patients. The percentage of non-responders was similar in DM and non-DM patients at T1 and T2 (P=0.45 and P=0.8, respectively) but increased significantly in DM patients at T3; 9 out of 11 (81.8%) DM patients had NAb levels lower than 0.3 µg/mL, in comparison to 16 out of 43 (37.2%) non-DM patients (chi-square=7.01, P=0.008). As the number of patients with DM in the studied sample was low, the conclusions on the effects of DM on the humoral response need further verification .
Patients on HDF showed an upward tendency in antibody titers compared with patients on HD (Fig. 2). The beneficial effect of HDF was significant at T3 (P=0.04) for NAb titers only (0.29 [0.1-0.55] vs. 0.84 [0.37-1.29] µg/mL in HD and HDF patients, respectively). Correction with ANCOVA did not show a significant effect of age or sex on NAb titers between the subgroups (P=0.94 and P=0.39 for age and sex, respectively). The proportion of non-responders at T3 was greater among HD patients (16/30, 53.3%) than among HDF patients (9/24, 37.5%), but the difference was not significant. Although the sample size may not be adequate to establish firm conclusions, a tendency for a better response in HDF patients has been shown.
Previous immunosuppressive treatment, administered either due to transplantation or primary glomerulonephritis, was not related to antibody levels. No difference was observed in the percentage of responders among previously immunosuppressed patients (data not shown).
We conducted lymphocyte subpopulation analysis in 33 patients at T0 and estimated the effect of each specific lymphocyte subtype on the response rate to vaccination against SARS-CoV-2. The T- and B-cell subsets before vaccination (T0) are shown in Supplemental Data Table S2.
Correlations between RBD titers and T-lymphocyte subsets are shown in Table 2. At T1, RBD titers were positively correlated with the CD4 RTE (CD4+CD45RA+CD31+) and naïve CD4 cell counts. However, after Bonferroni correction, only the correlation with CD4 RTE remained significant. No correlation was found between B-lymphocyte subpopulations and RBD titers or between T- and B-lymphocyte subpopulations and NAb titers at T1.
Moreover, no correlation was found between either the RBD or NAb titer at T2 with T- and B-lymphocyte subpopulations, except for a marginally significant positive correlation of NAb titers with the CD4+CD28–CD57+ percentage (BC=0.359, R2=0.129, P=0.05) at T2.
In the univariate linear regression analysis, RBD and NAb titers at T3 correlated positively with both the CD4 naïve T cell (BC=0.512, R2=0.262, P=0.006 and BC=0.803, R2=0.645, P<0.001, respectively) and CD4 RTE cell (BC=0.396, R2=0.156, P=0.04 and BC=0.455, R2=0.207, P=0.02, respectively) counts, whereas NAb titers showed a positive correlation with the CD8 RTE cell count (BC=0.416, R2=0.175, P=0.03) but not with the total naïve CD8 T cell count. There was a significant negative correlation between NAb titers and the percentage of a less differentiated CD4 T cell subset, CD4+CD28+CD57– T cells (BC=-0.454, R2=0.206, P=0.02), whereas both RBD and NAb levels showed a positive correlation with the percentage of a highly differentiated subtype of CD4 cells, CD4+CD28–CD57+ T cells (BC=0.417, R2=0.174, P=0.03 and BC=0.748, R2=0.56, P<0.001, respectively).
In CD8+ T cell subpopulations, the expression of CD28 and CD57 did not have any significant impact on antibody levels. However, the count of the advanced differentiated subset of CD8+ T cells, TEMRA (CD8+CD45RA+CCR7–), showed a significant positive correlation with NAb titers at T3 (BC=0.395, R2=0.156, P=0.04) (Table 3).
Of note, after Bonferroni correction of the significance level, only the CD4 RTE count and CD4+CD28–CD57+ percentage remained significantly associated with NAb levels at T3.
Multivariate linear regression analysis for NAb levels at T3 was performed with the three most significant CD4 subsets as identified in univariate analyses. This linear model explained 80.4% of the variance in the dependent variable, NAb titer, at T3 (R2=0.804, P<0.001). The CD4+CD28–CD57+ T cell subset was the main independent variable predicting NAb titers at T3 (P<0.001), followed by naïve CD4 T cells (P=0.05). The contribution of the CD4+ RTE count was not significant. Moreover, multivariate linear regression analysis including the two most significant CD8+ T cell subsets, CD8 RTE and TEMRA, showed that only CD8 TERMA could significantly predict NAb titers at T3, explaining 30.7% of the variance among patients (R2=0.307, P=0.01) (Table 3). Age was also included in the multivariate analysis; however, its contribution was not significant (P=0.14). When sex was included in the multivariate model, it appeared to significantly contribute to antibody titers. Therefore, we performed multiple analyses for male and female patients separately. The contribution of naïve CD4+ and CD4+CD28–CD57+ T cells remained significant in the male patient subgroup (R2=0.64, P<0.001), whereas the model was not significant for female patients.
Similarly, univariate linear regression analysis for B-lymphocyte subsets revealed no correlation with NAb titers. However, there was a significant positive correlation between RBD titers at T3 and the proportion of naïve B cells (BC=0.417, R2=0.147, P=0.03) and a negative correlation with the proportion of memory B cells (BC=-0.399, R2=0.159, P=0.04), as shown in Table 4. However, none of the above B-lymphocyte subpopulations emerged as significant independent parameters explaining NAb titers in the multivariate linear regression analysis (R2=0.207, P=0.06).
In this prospective observational study, we evaluated the humoral response of dialysis patients to vaccination against SARS-CoV-2 with the BNT162b2 vaccine and determined the clinical and immunological parameters that may affect this response. One month after the second dose, 17% of our patients did not achieve a sufficient response to reach seroconversion, as designated by NAb titers against SARS-CoV-2. Four months after the second dose, this proportion increased further to 46.3%. As similar results have been described previously, the low rate of response in ESRD patients is alerting and requires further investigation to elucidate the possible implicated parameters [5, 6]. Age and concomitant DM are the main clinical factors affecting the humoral immune response [6]. The negative impact of advanced age on NAb titers was evident early, even during the first and second months after complete vaccination, whereas DM had an indolent and delayed effect, as non-DM patients were more likely to maintain seroconversion 4 months after vaccination. It remains uncertain whether the presence of DM affects patients’ immune response to vaccination; however, the coexistence of DM and ESRD has a devastating effect on the immunogenicity rate [7, 8]. The dialysis method also affected the response rate, as patients on online HDF showed longer seroconversion than those on low-flux HD. Advanced clearance of inflammatory cytokines maintained by HDF may reduce systemic inflammation and improve the immunological response [9].
Immune disturbances in patients undergoing dialysis have been established over the last few decades. Most studies agree that patients undergoing dialysis are characterized by severe naïve T cell lymphopenia, with reduced thymic output and an increased proportion of senescent lymphocyte subsets, characterized as T lymphocytes lacking the CD28 co-stimulatory molecule and expressing CD57, a cytotoxic antigen [10]. These changes resemble those that occur during the normal aging process and have been attributed to deregulation of cytokine production and retention, accelerated naïve T cell apoptosis, gut barrier dysfunction, and enteric dysbiosis, leading to advanced antigenic stimuli [4, 11].
In our dialysis patients, an adequate response to vaccination against SARS-CoV-2 correlated positively with the naïve T helper cell count. Similar results have been reported in primates and healthy individuals [12]. Aging, as well as ESRD, has a detrimental effect on naïve T cell populations; however, a synergistic effect of both parameters on reducing naïve T cells has not been proven [13]. This is in accordance with our multivariate analysis results, in which age was not proven to be a significant contributor to antibody levels.
The percentage of senescent phenotypes, namely, CD4+CD28–CD57+ and CD8 TEMRA subtypes, was also positively correlated with the preservation of seroconversion at 4 months after vaccination. This effect was evident in the univariate analysis and confirmed in the multivariate analysis, further supporting the significant and independent beneficial effect of the above advanced differentiated “senescent” lymphocyte subpopulations in the development of the immune response to vaccination. This is a novel result, and although there are no sufficient data in the literature regarding the impact of immunosenescence in response to vaccination, the beneficial effect seems to be completely unpredictable.
We speculated that this discrepancy may involve activation of concomitant immune reactions related to terminally differentiated populations. In 2018, Wagner, et al. [14] showed that older individuals had an impaired humoral response to vaccination against Japanese encephalitis virus, along with concomitant naïve T cell lymphopenia and an increased percentage of regulatory T cells (Tregs) before vaccination.
Although the measurement of Tregs was not included in our initial protocol, based on previous results that emphasized their role in the immune response after vaccination, we retrospectively performed a correlation analysis between these two subsets, CD4+CD28–CD57+ and CD8 TEMRA, with the Treg population (using our database system on 150 ESRD patients on dialysis for >1 year). Tregs were significantly reduced in these patients [15]; moreover, we found a significant negative correlation between the number of Tregs and both the CD4+CD28–CD57+ and CD8 TEMRA subsets (Supplemental Data Fig. S2), supporting the hypothesis that these specific T cell subsets have an indirect beneficial effect on the humoral response, possibly due to the consequent reduction of Tregs [16].
Increased numbers of CD28– and CD8 TEMRA cells may force the upregulation of Th17 cells through increased interleukin (IL)-1 and IL-6 and reduced IL-2 production [17, 18]. Th17 cells not only antagonize Tregs but also contribute to antibody production through the production of the cytokine IL-21, consequently enhancing the conversion of B cells into plasma cells [19].
Moreover, the main difference between dialysis patients and the healthy aged population is their tendency to retain intact Th1 cells, in accordance with an impaired Th2 response [20-22]. Vitamin D deficiency appears to play a pivotal role in shifting toward Th1 and Th17 responses [23, 24]. This CD4 activation pattern was previously demonstrated in dialysis patients [25, 26] and was also shown to play a major role in immunity against SARS-CoV-2 infection [27, 28] and vaccination with mRNA vaccines [29].
The repressive role of Tregs in Ig production by B cells [30] and the response to booster vaccination [31] have been described in healthy individuals as well as in a small cohort of dialysis patients [32].
In contrast to our results showing no significant association between CD8+CD28– cells and the response to SARS-CoV-2 vaccination, previous studies on the response rate to influenza vaccination showed that elimination of CD28 molecules on CD8 cells was associated with a worse immune response [33-35], whereas results on CD4+CD28– cells are restricted [36]. The patients involved in these previous studies had already experienced contact with influenza virus, in contrast to our patients, who were inexperienced with SARS-CoV-2 at the time of vaccination. This could affect seroconversion rates, as repeated vaccination against influenza can affect the immune response [37, 38].
The total B-lymphocyte count did not seem to play a significant role in the response to vaccination, as this population was not associated with antibody titers. Naïve and memory B cells have direct beneficial and detrimental effects, respectively. A previous study has reported similar results [39]. The B-cell memory subset is greatly heterogeneous and incurs quantitative, but mainly qualitative, changes during aging and ESRD. Increased numbers of a proinflammatory B memory cell subpopulation, bearing the phenotype CD11c+T-bet+CD21–, have been described in older individuals and in those with several chronic inflammatory conditions and are associated with poor influenza-specific antibody production [40].
Our study confirmed the detrimental effects of advanced age and comorbidities such as DM in response to anti-SARS-CoV-2 vaccination in dialysis patients. Furthermore, this study demonstrated the beneficial role of CD4– naïve and also of CD4+CD28–CD57+ and CD8 TEMRA subsets in the response rate after vaccination, representing a novel yet completely unexpected result. Indirect evidence suggests the possible implication of additional and even challenging mechanisms such as the downregulation of Tregs, which needs to be further investigated. We recognize that this study has certain limitations. The number of patients included was limited, and hence, our findings should be validated in further studies. Moreover, the enrollment of patients on HD or HDF was not blinded, and consequently, the conclusions on HDF superiority regarding response to vaccination may be biased. Finally, wider lymphocyte phenotyping with concomitant functional studies is required to elucidate the mechanisms underlying the under-responsiveness to vaccination of patients with ESRD.
To our knowledge, this is the first study demonstrating this phenomenon, which supports that advanced differentiated lymphocytes are not always harmful. Our results further indicate that immune system disturbances in ESRD differ from those associated with aging or chronic inflammatory disease.
Notes
AUTHOR CONTRIBUTIONS
Stangou M, Fylaktou A, and Lioulios G: conception and study design; Stangou M and Papagianni A: manuscript revision and approval; Fylaktou A, Xochelli A, Asouchidou D, and Nikolaidou V: laboratory processing; Tsouchnikas I, Giamalis P, Stai S, Christodoulou M, and Lioulios G: data collection and interpretation; Lioulios G and Stangou M: manuscript drafting. All authors reviewed and approved the final version of the manuscript.
REFERENCES
1. Islam N, Jdanov DA, Shkolnikov VM, Khunti K, Kawachi I, White M, et al. Effects of covid-19 pandemic on life expectancy and premature mortality in 2020: time series analysis in 37 countries. BMJ. 2021; 375:e066768. DOI: 10.1136/bmj-2021-066768. PMID: 34732390. PMCID: PMC8564739.

2. Zsichla L, Müller V. 2023; Risk factors of severe COVID-19: a review of host, viral and environmental factors. Viruses. 15:175. DOI: 10.3390/v15010175. PMID: 36680215. PMCID: PMC9863423.

3. Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V. 2020; COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 16:e1008762. DOI: 10.1371/journal.ppat.1008762. PMID: 32822426. PMCID: PMC7444525.

4. Lioulios G, Fylaktou A, Papagianni A, Stangou M. 2021; T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin Immunol. 225:108685. DOI: 10.1016/j.clim.2021.108685. PMID: 33549833.

5. Cancro MP, Tomayko MM. 2021; Memory B cells and plasma cells: the differentiative continuum of humoral immunity. Immunol Rev. 303:72–82. DOI: 10.1111/imr.13016. PMID: 34396546.

6. Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. 2021; Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 16:1037–42. DOI: 10.2215/CJN.03500321. PMID: 33824157. PMCID: PMC8425628.

7. Chee YJ, Tan SK, Yeoh E. 2020; Dissecting the interaction between COVID-19 and diabetes mellitus. J Diabetes Investig. 11:1104–14. DOI: 10.1111/jdi.13326. PMID: 32558211. PMCID: PMC7323255.

8. Yan Z, Yang M, Lai CL. 2021; COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines. 9:1097. DOI: 10.3390/vaccines9101097. PMID: 34696205. PMCID: PMC8539110.

9. den Hoedt CH, Bots ML, Grooteman MP, van der Weerd NC, Mazairac AH, Penne EL, et al. 2014; Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 86:423–32. DOI: 10.1038/ki.2014.9. PMID: 24552852.

10. Lioulios G, Fylaktou A, Xochelli A, Sampani E, Tsouchnikas I, Giamalis P, et al. 2022; Clustering of end stage renal disease patients by dimensionality reduction algorithms according to lymphocyte senescence markers. Front Immunol. 13:841031. DOI: 10.3389/fimmu.2022.841031. PMID: 35615367. PMCID: PMC9126282.

11. Vaziri ND. 2014; Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig Dis Sci. 59:2020–2. DOI: 10.1007/s10620-014-3287-z. PMID: 25038737.

12. Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, et al. 2010; Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 184:6739–45. DOI: 10.4049/jimmunol.0904193. PMID: 20483749. PMCID: PMC3504654.

13. Freitas GRR, da Luz Fernandes M, Agena F, Jaluul O, Silva SC, Lemos FBC, et al. 2019; Aging and end stage renal disease cause a decrease in absolute circulating lymphocyte counts with a shift to a memory profile and diverge in Treg population. Aging Dis. 10:49–61. DOI: 10.14336/AD.2018.0318. PMID: 30705767. PMCID: PMC6345336.

14. Wagner A, Garner-Spitzer E, Jasinska J, Kollaritsch H, Stiasny K, Kundi M, et al. 2018; Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep. 8:9825. DOI: 10.1038/s41598-018-28111-8. PMID: 29959387. PMCID: PMC6026142.

15. Sampani E, Daikidou DV, Lioulios G, Xochelli A, Mitsoglou Z, Nikolaidou V, et al. 2021; CD28null and regulatory T cells are substantially disrupted in patients with end-stage renal disease due to diabetes mellitus. Int J Mol Sci. 22:2975. DOI: 10.3390/ijms22062975. PMID: 33804135. PMCID: PMC8001943.

16. Bergström M, Joly AL, Seiron P, Isringhausen S, Modig E, Fellström B, et al. 2015; Immunological profiling of haemodialysis patients and young healthy individuals with implications for clinical regulatory T cell sorting. Scand J Immunol. 81:318–24. DOI: 10.1111/sji.12287. PMID: 25737071.

17. Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. 1998; Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev. 165:287–300. DOI: 10.1111/j.1600-065X.1998.tb01246.x. PMID: 9850868.

18. Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. 2018; Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 17:e12675. DOI: 10.1111/acel.12675. PMID: 29024417. PMCID: PMC5770853.
19. Spolski R, Leonard WJ. 2014; Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 13:379–95. DOI: 10.1038/nrd4296. PMID: 24751819.

20. Zamauskaite A, Perez-Cruz I, Yaqoob MM, Madrigal JA, Cohen SB. 1999; Effect of renal dialysis therapy modality on T cell cytokine production. Nephrol Dial Transplant. 14:49–55. DOI: 10.1093/ndt/14.1.49. PMID: 10052476.

21. Libetta C, Rampino T, Dal Canton A. 2001; Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. Am J Kidney Dis. 38:286–95. DOI: 10.1053/ajkd.2001.26092. PMID: 11479154.

22. Mansouri L, Nopp A, Jacobson SH, Hylander B, Lundahl J. 2017; Hemodialysis patients display a declined proportion of Th2 and regulatory T cells in parallel with a high interferon-γ profile. Nephron. 136:254–60. DOI: 10.1159/000471814. PMID: 28380480.

23. Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, et al. 2010; Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol. 39:490–7. DOI: 10.3109/03009741003781951. PMID: 20615161.

24. Charoenngam N, Holick MF. 2020; Immunologic effects of vitamin D on human health and disease. Nutrients. 12:2097. DOI: 10.3390/nu12072097. PMID: 32679784. PMCID: PMC7400911.

25. Agüera ML, Hurtarte AR, Álvarez de Lara MA, Aumente MD, Carmona A, Carracedo J, et al. 2016; Donor-specific antibodies after starting hemodialysis in nonrenal solid organ transplant recipients: role of TH17. Transplant Proc. 48:2920–3. DOI: 10.1016/j.transproceed.2016.10.003. PMID: 27932108.

26. Lang CL, Wang MH, Hung KY, Hsu SH, Chiang CK, Lu KC. 2014; Correlation of interleukin-17-producing effector memory T cells and CD4+CD25+ Foxp3 regulatory T cells with the phosphate levels in chronic hemodialysis patients. ScientificWorldJournal. 2014:593170. DOI: 10.1155/2014/593170. PMID: 24558316. PMCID: PMC3914580.
27. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. 2020; Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 11:3434. DOI: 10.1038/s41467-020-17292-4. PMID: 32632085. PMCID: PMC7338513.

28. Gil-Etayo FJ, Garcinuño S, Utrero-Rico A, Cabrera-Marante O, Arroyo-Sanchez D, Mancebo E, et al. 2022; An early Th1 response is a key factor for a favorable COVID-19 evolution. Biomedicines. 10:296. DOI: 10.3390/biomedicines10020296. PMID: 35203509. PMCID: PMC8869678.

29. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. 2021; Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 54:2133–42.e3. DOI: 10.1016/j.immuni.2021.08.001. PMID: 34453880. PMCID: PMC8361141.

30. Lim HW, Hillsamer P, Banham AH, Kim CH. 2005; Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 175:4180–3. DOI: 10.4049/jimmunol.175.7.4180. PMID: 16177055.
31. Garner-Spitzer E, Wagner A, Paulke-Korinek M, Kollaritsch H, Heinz FX, Redlberger-Fritz M, et al. 2013; Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol. 191:2426–36. DOI: 10.4049/jimmunol.1300293. PMID: 23872054.

32. Mathew RO, Mason DL, Song R, Tryniszewski T, Kennedy JS. 2016; Role of T-regulatory cells in the response to hepatitis B vaccine in hemodialysis patients. Hemodial Int. 20:242–52. DOI: 10.1111/hdi.12326. PMID: 26104830.

33. Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. 2001; Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 75:12182–7. DOI: 10.1128/JVI.75.24.12182-12187.2001. PMID: 11711609. PMCID: PMC116115.

34. Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, et al. 2002; Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 168:5893–9. DOI: 10.4049/jimmunol.168.11.5893. PMID: 12023394.

35. Alonso Arias R, Moro-García MA, Echeverría A, Solano-Jaurrieta JJ, Suárez-García FM, López-Larrea C. 2013; Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. J Virol. 87:4486–95. DOI: 10.1128/JVI.02425-12. PMID: 23388717. PMCID: PMC3624366.

36. Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. 2013; Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 31:685–90. DOI: 10.1016/j.vaccine.2012.11.041. PMID: 23196209.

37. Künzel W, Glathe H, Engelmann H, Van Hoecke C. 1996; Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 14:1108–10. DOI: 10.1016/0264-410X(96)00061-8. PMID: 8911005.

38. Pyhälä R, Kumpulainen V, Alanko S, Forsten T. 1994; HI antibody kinetics in adult volunteers immunized repeatedly with inactivated trivalent influenza vaccine in 1990-1992. Vaccine. 12:947–52. DOI: 10.1016/0264-410X(94)90039-6. PMID: 7975836.

39. Siegrist CA, Aspinall R. 2009; B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 9:185–94. DOI: 10.1038/nri2508. PMID: 19240757.

40. Gustafson CE, Kim C, Weyand CM, Goronzy JJ. 2020; Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 145:1309–21. DOI: 10.1016/j.jaci.2020.03.017. PMID: 32386655. PMCID: PMC7198995.

Fig. 1
Antibody titers at T1, T2, and T3. (A) Anti-RBD values are presented in units of AU/mL, and (B) NAb values are presented in units of μg/mL. Total number of patients and numbers of responders at each time point are shown in the table below. Responders were defined as patients with an RBD titer >1.0 AU/mL and an NAb titer >0.3 μg/mL. **P<0.005.
Abbreviations: T1, 30 days after vaccination; T2, 60 days after vaccination; T3, 120 days after vaccination; RBD, receptor-binding domain; NAb, neutralizing antibody.
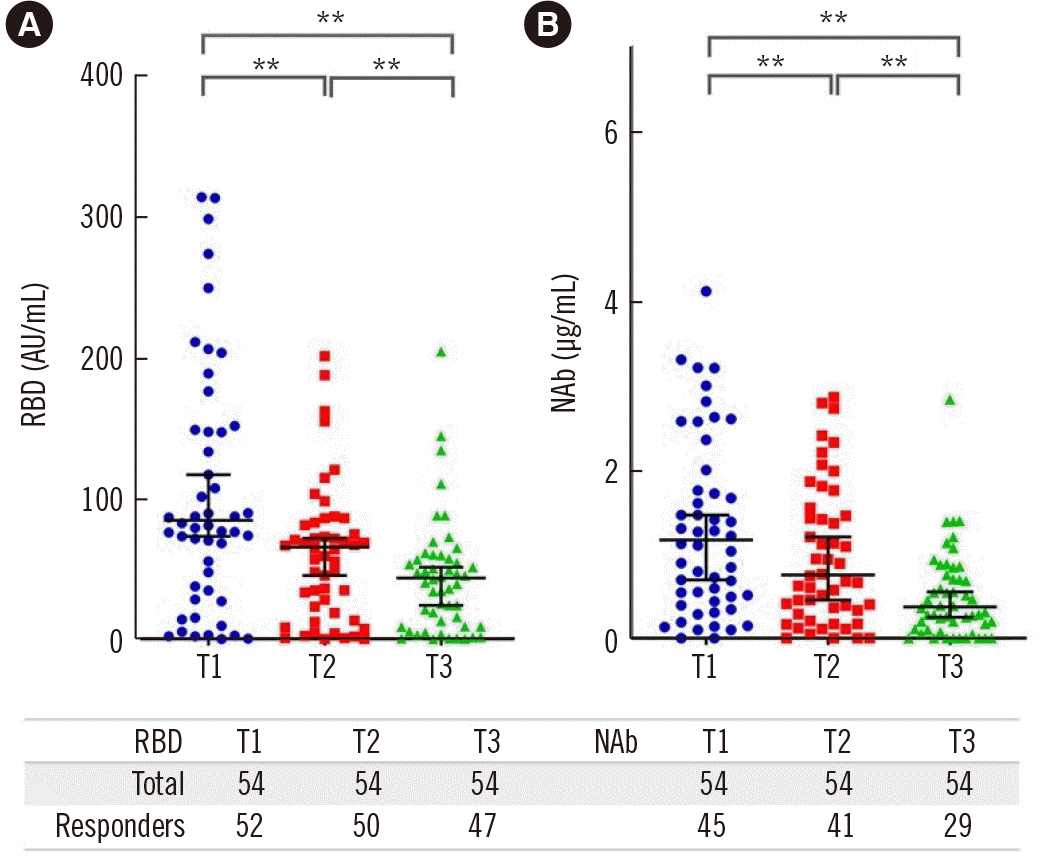
Fig. 2
Antibody titers at T1, T2, and T3. (A) Anti-RBD (AU/mL) in patients without DM (non-DM) in comparison to that in patients with DM (positive RBD titer >1.0 AU/mL in 41/43, 39/43, and 38/43 non-DM patients and in 11/11, 11/11, and 9/11 DM patients at T1, T2, and T3, respectively). (B) NAbs (μg/mL) in non-DM patients and DM patients (positive NAb titer > 0.3 μg/mL in 35/43, 32/43, and 27/43 non-DM patients and in 9/11, 9/11, and 2/11 DM patients at T1, T2, and T3, respectively). (C) Anti-RBD (AU/mL) in patients undergoing HD and HDF (positive RBD titer >1.0 AU/mL in 29/30, 29/30, and 27/30 HD patients and in 23/24, 21/24, and 20/24 HDF patients at T1, T2, and T3, respectively). (D) NAbs (μg/mL) in patients undergoing HD and HDF (positive NAb titer >0.3 μg/mL in 24/30, 21/30, and 14/30 HD patients and in 20/24, 20/24, and 15/24 HDF patients at T1, T2, and T3, respectively). *P<0.05, **P<0.005.
Abbreviations: T1, 30 days after vaccination; T2, 60 days after vaccination; T3, 120 days after vaccination; RBD, receptor-binding domain; NAb, neutralizing antibody; DM, diabetes mellitus; HD, hemodialysis; HDF, hemodiafiltration; NS, not significant (P>0.05).
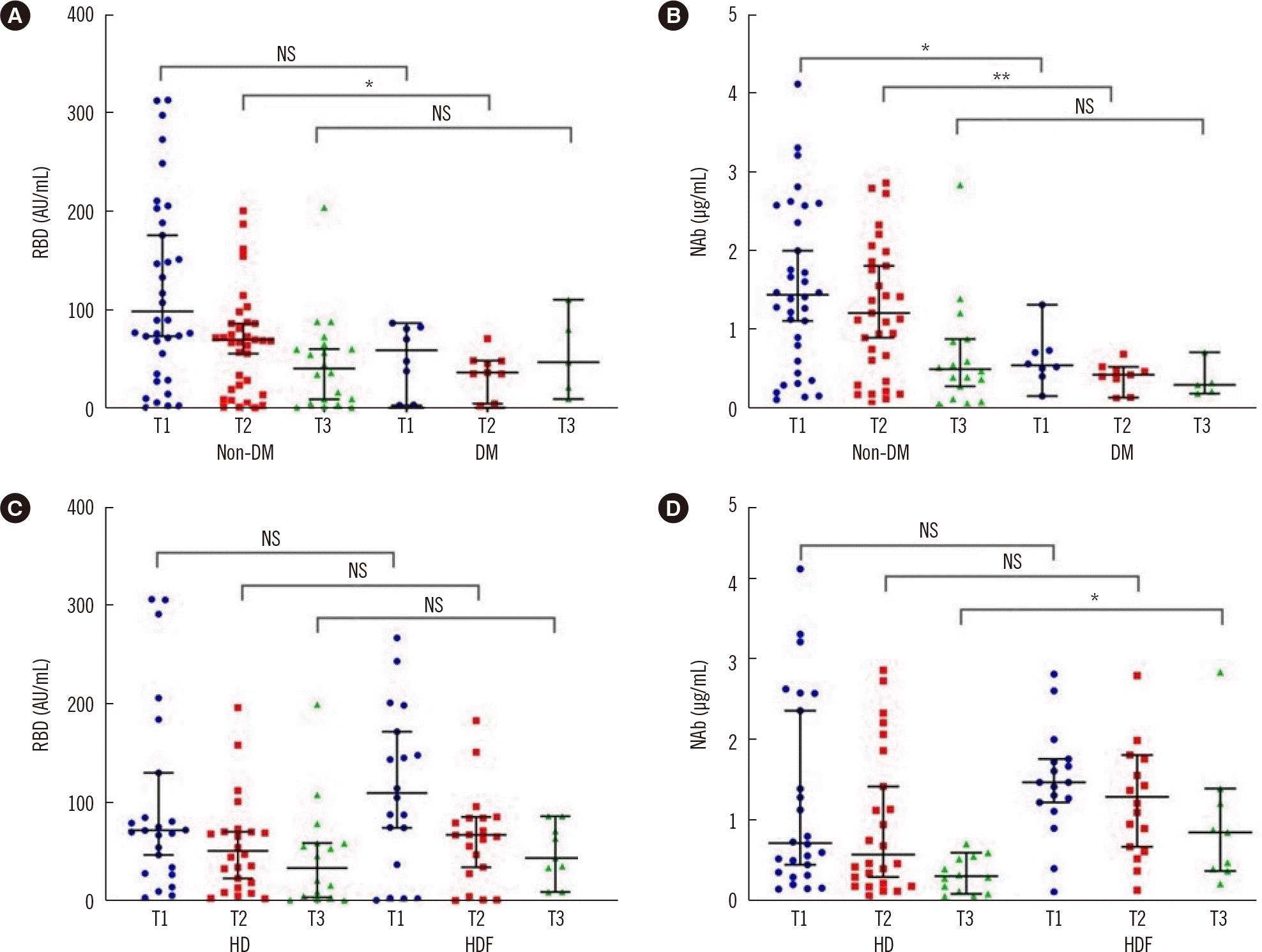
Table 1
Patient demographics
Table 2
Univariate linear regression of T-cell subsets associated with RBD titers at T1 and T3
| T-cell subset | Univariate linear regression at T1 | Univariate linear regression at T3 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| BC | R2 | 95% CI | P | BC | R2 | 95% CI | P | |
| CD4+ naïve | 0.446 | 0.199 | 0.212–1.840 | 0.01 | 0.512 | 0.262 | 0.066–0.364 | 0.006 |
| CD4+CD31+ | 0.504 | 0.254 | 0.802–4.151 | 0.005* | 0.396 | 0.156 | 0.014–0.628 | 0.04 |
| CD4+ TEMRA | 0.224 | 0.050 | -2.438–9.264 | 0.24 | 0.237 | 0.056 | -0.406–1.588 | 0.23 |
| CD4+CD28+CD57–% | -0.071 | 0.005 | -12.34–8.579 | 0.71 | -0.204 | 0.042 | -3.697–1.210 | 0.30 |
| CD4+CD28–CD57+% | 0.130 | 0.017 | -8.556–17.038 | 0.50 | 0.417 | 0.174 | 0.374–6.902 | 0.03 |
| CD8+ naïve | 0.191 | 0.036 | -0.534–1.569 | 0.32 | 0.142 | 0.020 | -0.118–0.244 | 0.48 |
| CD8+CD31+ | 0.225 | 0.051 | -0.449–1.712 | 0.24 | 0.208 | 0.043 | -0.089–0.279 | 0.29 |
| CD8+ TEMRA | 0.247 | 0.061 | -0744–3.453 | 0.19 | 0.246 | 0.047 | -0.196–0.651 | 0.28 |
| CD8+CD28+CD57–% | 0.102 | 0.010 | -5.141–8.753 | 0.55 | 0.180 | 0.032 | -0.764–1.985 | 0.36 |
| CD8+CD28–CD57+% | 0.049 | 0.002 | -8.866–6.917 | 0.80 | 0.022 | 0.000 | -1.682–1.510 | 0.91 |
Table 3
Univariate and multivariate analyses of T-cell subsets associated with NAb titers at T3
| T-cell subset | Univariate linear regression at T1 | Univariate linear regression at T3 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| BC | R2 | 95% CI | P | BC | PC | 95% CI | P | |
| CD4+ naïve | 0.803 | 0.645 | 0.005–0.009 | < 0.001* | 0.404 | 0.236 | 0.001–0.006 | 0.05 |
| CD4+CD31+ | 0.455 | 0.207 | 0.002–0.014 | 0.02 | 0.188 | 0.130 | -0.002–0.008 | 0.17 |
| CD4+ TEMRA | 0.274 | 0.075 | -0.006–0.035 | 0.16 | - | - | - | - |
| CD4+CD28+CD57–% | -0.454 | 0.206 | -0.08–0.008 | 0.02 | - | - | - | - |
| CD4+CD28–CD57+% | 0.748 | 0.560 | 0.055–0.118 | < 0.001* | 0.528 | 0.394 | 0.032–0.091 | < 0.001 |
| CD8+ naïve | 0.286 | 0.046 | -0.001–0.006 | 0.14 | - | - | - | - |
| CD8+CD31+ | 0.418 | 0.175 | 0.001–0.008 | 0.03 | 0.217 | 0.190 | -0.002–0.016 | 0.27 |
| CD8+ TEMRA | 0.395 | 0.156 | 0–0.015 | 0.04 | 0.414 | 0.361 | 0–0.016 | 0.04 |
| CD8+CD28+CD57–% | -0.078 | 0.006 | -0.031–0.021 | 0.70 | ||||
| CD8+CD28–CD57+% | 0.264 | 0.070 | -0.009–0.047 | 0.18 | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download