Abstract
With the projected increase in the global population, current healthcare delivery models will face severe challenges. Rural and remote areas, whether in developed or developing countries, are characterized by the same challenges: the unavailability of hospitals, lack of trained and skilled staff performing tests, and poor compliance with quality assurance protocols. Point-of-care testing using artificial intelligence (AI) is poised to be able to address these challenges. In this review, we highlight some key areas of application of AI in point-of-care testing, including lateral flow immunoassays, bright-field microscopy, and hematology, demonstrating this rapidly expanding field of laboratory medicine.
Improved living standards due to technological advances have led to a larger aging population globally. Furthermore, the world’s population is projected to reach 8.5 billion by 2030, 9.7 billion by 2050, and 11.2 billion by 2100 [1]. Current healthcare resources available to effectively cater to this burgeoning population cannot keep pace with this rate of increase and pose a challenge to public health programs worldwide.
Point-of-care (POC) testing, where patients are tested and treated at the hospital bedside, in pharmacies, at community centers, or in their own homes, provides a workable healthcare solution. One of the challenges in performing POC testing is ensuring that the results are reliable and correctly interpreted. This requires properly trained users and quality assurance practices. Artificial intelligence (AI) is making important contributions to POC testing and is expected to help resolve many of the challenges faced by healthcare workers and in the widespread application of direct-to-consumer testing. In this review, we highlight some important examples where AI facilitates developments in this rapidly expanding field.
AI is a wide-ranging discipline that includes machine learning (ML), robotics, and visual computation. However, only those aspects of AI that are relevant to POC testing are discussed herein. ML, a subset of artificial learning, is used to create algorithms for solving problems and building “intelligent machines.” Hence, ML is the “brain” of AI. The most common ML algorithm currently used in POC testing is “supervised learning,” where the machine is given “inputs” and associated “outputs.” When a new input is provided, the memory is scanned to identify the associated output. In the 1960s and 1970s, ML algorithms relied on linear “if–then” relationships [2]. However, as our understanding of the human brain function has improved, scientists have attempted to better mimic it in ML, leading to the development of neural networks (NNs) [2].
An artificial NN (ANN) is composed of the following node layers: an input layer, hidden layer, and output layer. Each node is connected to another node and associated with a particular weight and threshold [3]; i.e., positive and negative weights represent individual responses to an input that results in an output [2]. If the output of a node exceeds a particular threshold, the node is activated and signals are sent to the next node; otherwise, no signals are sent [3]. Hence, an ANN is an example of ML that uses information and helps the computer generate an output based on stored examples or previous encounters [4]. Convolutional NNs (CNNs) are best suited to work with image, speech, or audio signal inputs. They have three main types of layers: convolutional, pooling, and fully connected. In summary, as image data progress through the layers of a CNN, the model starts to recognize larger elements or shapes of the object until it finally identifies the target object [3].
LFIAs are among the most common diagnostic platforms for POC testing because they can provide results in as little as 10 minutes. LFIAs are easy to perform, user-friendly, and possess cost-effective features that satisfy the “ASSURED” WHO benchmark of POC tests (A=affordable, S=sensitive, S=specific, U=user-friendly, R=robust, E=equipment-free, D=deliverable to those who need them) [5].
LFIAs typically use gold nanoparticles (GNPs) conjugated with antibodies that detect antigens of interest. However, because interpretation of LFIA results relies on the ability to observe the control and test lines visually, there are some limitations to sensitivity. Replacing GNPs with fluorophores has improved the sensitivity of LFIAs; however, their use has been limited by platform instability when stored at room temperature [6] and their susceptibility to photobleaching [7]. The use of quantum dots has improved photostability, resulting in large molar extinction coefficients and high fluorescent quantum yield [8]. However, quantum dots exhibit high autofluorescence [9]. Another limitation of these optical testing platforms is that subsurface signals are not measured, and result interpretation is based on red, green, and blue light or gray-scale scanning, which limits their sensitivity and accuracy [10]. Yan, et al. [8] used magnetic nanoparticles conjugated to antibodies and measured the magnetic signals of the analytes human chorionic gonadotropin (hCG), cardiac troponin I, creatine kinase isoenzyme MB, and myoglobin using a magnetic immunoassay reader. This method enabled the detection of signals in the entire test zone regardless of the opacity or high noise-to-signal ratio [8]. Moreover, for weak magnetic signals (10−7–10−4 Oe) on test strips, they used a novel data-processing method based on a support vector machine classifier and custom waveform reconstruction, thereby significantly improving the sensitivity and accuracy of the test [8]. hCG was quantitatively detected in the range of 1–1,000 mIU/mL with a detection limit of 0.014 mIU/mL. This is a very low concentration, considering that laboratory instruments typically have a cut-off <5 mIU/mL [8].
The key step in using AI for LFIAs is building an image library. Turbé, et al. [11] built an image library using two different HIV LFIAs. The workflow was the same for both LFIAs: a drop of blood from a fingertip was applied to a pad, buffer was added to help movement of the specimen across the paper or solid support membrane by capillary action, and after 10–40 minutes, antigen–antibody reactions produced visible lines corresponding to “Test” and “Control” lines indicating verified positive or negative test results [11]. Although LFIAs are attractive for POC testing, the accuracy of result interpretation can vary from 80% to 97%, depending on the training and experience of the user, as the test lines can sometimes be faint or the user may suffer from color blindness [11]. To counter this inadequacy of HIV LFIAs, a deep-learning algorithm was developed. Each patient was tested using both POC LFIAs; images were captured using a Samsung tablet (SM-P585) 8M Pixels camera (f1/9) with autofocus capability (Samsung, Seoul, Korea) integrated with a native Android camera application and sent to the mobile health system. A pilot field study of the algorithms deployed as a mobile application demonstrated increased sensitivity (97.8%) and specificity (100%) compared to those achieved with traditional visual interpretation by humans, including experienced nurses and newly trained community health workers, and reduced the numbers of false positives and false negatives [11].
Malaria is an infectious disease caused by parasites of the Plasmodium genus transmitted via a female Anopheles mosquito vector [12]. In 2019, the WHO estimated that there were 229 million cases of malaria worldwide, with 409,000 deaths [13]. Children under the age of 5 years are the most vulnerable group, accounting for 67% (274,000) of all malaria-related deaths worldwide. African regions account for an estimated 94% of all cases [13], with a predominance of Plasmodium falciparum infection [14].
Microscopic identification of malarial parasites in a peripheral blood smear is historically the gold standard for malaria diagnosis and has well-established quality assurance practices; thus, properly trained healthcare workers can correctly quantify parasitemia and identify parasite species [15]. Microscopy generally has a detection limit of 20 parasites/µL [16], and in patients with clinical malaria, microscopic slide examination is associated with a diagnostic sensitivity of 75% [16]. However, the detection limit is lower in patients with non-P. falciparum malaria, low parasitemia, or partial immunity [15]. As skilled microscopists or properly working microscopes may not always be available in resource-limited settings, there has been a drive to develop better diagnostic methods that can be performed easily in remote, rural healthcare settings.
An automated microscopic analysis system comprises hardware that captures images and software that applies an algorithm to interpret the images and make diagnostic decisions. As mentioned above, a CNN is an ML model based on the human visual system that uses the mathematical operation of convolution to interpret captured images and has been highly successful in image-related tasks [17].
EasyScan Go is an automated microscopy system developed by Motic (Hong Kong, China) that captures images and uses the CNN algorithm to interpret the images and make a diagnostic decision. Das, et al. [18] compared the results obtained by experienced microscopists with those obtained automatically with EasyScan Go in diagnosing malarial blood smear slides. The diagnostic sensitivity of EasyScan Go was 91.1% (95% confidence interval [CI]: 88.9%–92.7%), and the specificity was 75.6% (95% CI: 73.1%–78.0%). With good-quality slides, the sensitivity was similar (89.1%, 95% CI: 86.2%–91.5%), but the specificity increased to 85.1% (95% CI: 82.6%–87.4%) [18]. Slide quality was a significant contributor to both specificity and sensitivity. Although there is still room for improvement, especially for estimations in cases of low parasitemia and parasite density, with current research efforts by various groups, AI is expected to significantly improve malaria diagnosis.
Schistosomiasis is a neglected tropical disease caused by the flatworm Schistosoma spp., affecting approximately 236.6 million people, mainly in Africa [19]. Diagnosis is made using bright-field microscopy to identify eggs in a urine sample. Operator skills and experience are important because mild infections with low egg secretion can easily be missed [20].
Oyibo, et al. [20] designed a schistoscope, which is a low-cost, high-quality digital microscope that can also function as a slide scanner. They used an CNN model to identify the images using a data bank of 5,000 Schistosoma haematobium egg images captured from spiked and clinical urine specimens collected in field settings. The schistoscope was constructed using easily accessible parts. The optical system comprises a Raspberry Pi High-Quality Camera Module V2.1 (Alast Corp., Rowland Heights, CA, USA) equipped with a Sony IMX477R stacked, back-illuminated sensor (12.3-megapixel resolution, 7.9-mm sensor diagonal, and 1.55×1.55-µm sensor pixel size) (Arducam Technology Co. Ltd., Hong Kong, China). To visually identify Schistosoma eggs, they used a 4× magnification objective; however, the device was designed such that the objective could be easily interchanged with a 20× magnification objective [20]. The CNN algorithm clearly identified eggs in the images, showing that the image quality was suitable for automatic detection of Schistosoma eggs in line with the current diagnostic reference standard. High-quality microscopic images of S. haematobium, Schistosoma mansoni, and hookworm eggs were captured by microscopists using this device, and the eggs were clearly identified in digital images [20]. In a preliminary study, they found that the schistoscope had 80% sensitivity, which satisfied the WHO Target Product requirement for the diagnosis of schistosomiasis [21].
The routine requirement of a complete blood count (CBC) in hematology poses a challenge to developers of POC testing devices. This is mainly because a CBC requires not only counting cells but also differentiating cell size and morphology, which in a blood specimen can span a spectrum of cell maturity [22]. HemoScreen, designed by PixCell Medical Technologies, Ltd. (Yokne’am lllit, Israel), is the first Food and Drug Administration-approved POC hematology analyzer [23] that overcomes the challenges often presented in POC hematology testing. For the testing of cells in fluids, the flow must be streamlined into single-particle streams. Flow cytometry uses this technique to allow the scattering of fluorescent light for cell counting and morphological analysis.
HemoScreen consists of an analytic device and a disposable cartridge that contains all reagents required for testing. A part of the cartridge termed the “sampler” directly collects blood via a finger stick or from a venous specimen and is placed into the analytic device, where the blood is mixed with the reagents before entering a translucent chamber for optical analysis and enumeration [23]. Through a phenomenon known as viscoelastic focusing, also known as the Fahraeus–Lindqvist effect [24], blood cells migrate and concentrate at the centerline of small blood vessels or microchannels under laminar flow. This results in a single layer of cells, which aids in optical analysis [25]. For cellular analysis, HemoScreen uses machine-vision technology (image processing and analysis) rather than laser scattering or impedance [23]. Machine vision enables the accurate imaging of hundreds of flowing cells, capturing unique peculiarities that are later used in AI algorithms to identify individual cells and subtypes. Interference from cell debris and platelets can be identified to avoid erroneous reporting of the results. Hence, HemoScreen can be used by operators with minimal hematology training [23].
Sight Diagnostics Ltd. (Tel Aviv, Israel) developed the Sight OLO POC hematology analyzer that uses computer vision for image analysis [26]. Similar to HemoScreen, Sight OLO can use fingerstick and venous blood specimens, both of which are collected in potassium EDTA-coated capillary tubes. Hb testing requires a specimen volume of 17 μL, whereas cell staining for image analysis requires 10 μL of blood, which is mixed with a diluent and dried fluorescent stains. The red channel represents Hb, the green channel represents cytoplasmic staining, and the blue channel represents DNA nuclear staining.
A challenge in POC hematology is the ability to identify abnormal cells in blood smears. The process of preparing a monolayer blood smear is necessary to identify cell types. However, this requires technical skills that may not always be available at the POC during an emergency crisis or in remote rural areas.
Sight OLO circumvents this problem through a novel process in which diluted cells are drawn into the image chamber by capillary action, and the dimensions of the chamber are such that as the cells settle, a monolayer develops. These monolayer blood smears are rapidly imaged using the Sight OLO automated bright-field and fluorescence microscope that captures thousands of multispectral images of a single blood specimen based on optical and chemical signatures. Multispectral images are generated at five wavelengths: red (633 nm), green (577 nm), blue (460 nm), violet (405 nm), and ultraviolet (365 nm). These different illumination wavelengths allow the identification of erythrocytes, leukocytes, and platelets [26].
Furthermore, Sight OLO uses three different analysis workflows to count and characterize erythrocytes, leukocytes, and platelets. All three workflows involve a two-step process, in which a preliminary batch of candidates is identified before being further analyzed for definite identification [26]. Bright-field images are used to identify erythrocytes, which may still overlap if they are too close to each other. Therefore, once the candidates are identified, they are screened for overlap and split into individual cells based on morphological features. Erythrocyte images are further characterized using CNN algorithms to estimate cell properties such as mean cell Hb, mean cell volume, and mean cell Hb concentration [26]. Leukocytes are identified using both bright-field and fluorescent channels. The nucleus and cytoplasm are first identified in each cell and then further characterized based on different features, using a computer. ML is used to analyze these features and classify different leukocyte types as well as to identify abnormal cell types using classification algorithms. True platelets are identified by first filtering the candidates according to different morphological and intensity properties and then applying several CNN algorithms that are trained to accurately distinguish platelets from the background in different scenarios [26]. Table 1 compares the characteristics of the HemoScreen and Sight OLO devices.
Anemia is a serious health problem that affects approximately one third of the world’s population [27]. In sub-Saharan Africa, its prevalence reaches up to 91% in schoolchildren [28]. The consequences of anemia include poor birth outcomes, impaired cognitive and behavioral development, and decreased productivity in adults [27]. Anemia and sickle cell disease are associated with high mortality and morbidity in resource-limited countries, posing significant health problems. Recently, An, et al. [27] developed a POC microchip electrophoresis device that can measure both anemia and Hb variants using AI algorithms. The ANN-based ML algorithm measures the Hb concentration. Whole blood is diluted in a standard calibrator and electrophoresed on a cellulose acetate paper. After 10 minutes, the Hb is separated into subtypes according to the finer mass-to-charge ratio. The Hb concentration is then determined by comparing the Hb band intensity with the intensity of the standard calibrator. High-resolution images are captured of both the Hb band (red) and the standard calibrator band (blue), including all relevant pixel information. Each frame in the image video is sequentially split into the constituent red and blue channels. This information is then fed as the input feature vector to a trained ANN that examines the intensity ratio pattern and reports the corresponding Hb concentration (g/dL) (Fig. 1). The results are finally used to determine the anemia status of the patient. Using this system, anemia could be detected with 100% sensitivity and 92.3% specificity. Patients with sickle cell disease were identified with 100% sensitivity and specificity. Overall, this platform enables integrated anemia detection and Hb variant identification using a single POC testing device [27].
The use of AI in POC testing will bring about radical and permanent changes, in the same way information technology influenced laboratory medicine in the 1980s. The biggest advantage of AI in POC testing is its ability to perform the necessary diagnostic testing reliably and accurately without the need for skilled or trained personnel. This will significantly impact community healthcare and avoid the trend of population migration to urban areas in resource-limited countries, preventing unnecessary overcrowding and self-inflicted poverty, while improving the quality of life of those living in remote and rural areas.
Notes
REFERENCES
1. United Nations. Global issues. https://www.un.org/en/globalissues/population. Updated on Aug 2022.
2. Weka. Machine learning vs. neural networks (differences explained). https://www.weka.io/learn/machine-learning-gpu/machine-learning-vs-neural-networks. Updated on Sept 2021.
3. IBM. What are neural networks? https://www.ibm.com/topics/neural-networks. Updated on Dec 2022.
4. Western Governors University. Neural networks and deep learning explained. https://www.wgu.edu/blog/neural-networks-deep-learning-explained2003.html#openSubscriberModal. Updated on Mar 2020.
5. Kosack CS, Page AL, Klatser PR. 2017; A guide to aid the selection of diagnostic tests. Bull World Health Organ. 95:639–45. DOI: 10.2471/BLT.16.187468. PMID: 28867844. PMCID: PMC5578377.

6. Xu Y, Liu Y, Wu Y, Xia X, Liao Y, Li Q. 2014; Fluorescent probe-based lateral flow assay for multiplex nucleic acid detection. Anal Chem. 86:5611–4. DOI: 10.1021/ac5010458. PMID: 24892496.

7. Liu CY, Ma W, Gao ZY, Huang JY, Hou Y, Xu CL, et al. 2014; Upconversion luminescence nanoparticles-based lateral flow immunochromatographic assay for cephalexin detection. J Mater Chem C. 2:9637–42. DOI: 10.1039/C4TC02034K.

8. Yan W, Wang K, Xu H, Huo X, Jin Q, Cui D. 2019; Machine learning approach to enhance the performance of MNP-labeled lateral flow immunoassay. Nanomicro Lett. 11:7. DOI: 10.1007/s40820-019-0239-3. PMID: 34137967. PMCID: PMC7770769.

9. Wang CY, Hou F, Ma YC. 2015; Simultaneous quantitative detection of multiple tumor markers with a rapid and sensitive multicolor quantum dots based immunochromatographic test strip. Biosens Bioelectron. 68:156–62. DOI: 10.1016/j.bios.2014.12.051. PMID: 25562743.

10. Zhao Y, Yang M, Fu Q, Ouyang H, Wen W, Song Y, et al. 2018; A nanozyme- and ambient light-based smartphone platform for simultaneous detection of dual biomarkers from exposure to organophosphorus pesticides. Anal Chem. 90:7391–8. DOI: 10.1021/acs.analchem.8b00837. PMID: 29792679.

11. Turbé V, Herbst C, Mngomezulu T, Meshkinfamfard S, Dlamini N, Mhlongo T, et al. 2021; Deep learning of HIV field-based rapid tests. Nat Med. 27:1165–70. DOI: 10.1038/s41591-021-01384-9. PMID: 34140702. PMCID: PMC7611654.

12. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong'echa JM. 2011; Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 7:1427–42. DOI: 10.7150/ijbs.7.1427. PMID: 22110393. PMCID: PMC3221949.

13. WHO. World Malaria Report, 2020. https://www.who.int/publications/i/item/9789240015791. Updated on Nov 2020.
14. Belachew EB. 2018; Immune response and evasion mechanisms of Plasmodium falciparum parasites. J Immunol Res. 2018:6529681. DOI: 10.1155/2018/6529681. PMID: 29765991. PMCID: PMC5889876.
15. Wilson ML. 2013; Laboratory diagnosis of malaria: conventional and rapid diagnostic methods. Arch Pathol Lab Med. 137:805–11. DOI: 10.5858/arpa.2011-0602-RA. PMID: 23721276.

16. Ratnawati , Hatta M, Smits HL. 2008; Point-of-care testing for malaria outbreak management. Trans R Soc Trop Med Hyg. 102:699–704. DOI: 10.1016/j.trstmh.2008.04.018. PMID: 18513771.

17. LeCun Y, Bengio Y, Hinton G. 2015; Deep learning. Nature. 521:436–44. DOI: 10.1038/nature14539. PMID: 26017442.

18. Das D, Vongpromek R, Assawariyathipat T, Srinamon K, Kennon K, Stepniewska K, et al. 2022; Field evaluation of the diagnostic performance of EasyScan GO: a digital malaria microscopy device based on machine-learning. Malar J. 21:122. DOI: 10.1186/s12936-022-04146-1. PMID: 35413904. PMCID: PMC9004086.

19. WHO guideline on control and elimination of human schistosomiasis [Internet]. World Health Organization;Geneva: 2022. https://www.ncbi.nlm.nih.gov/books/NBK578389/.Updated on March, 2023.
20. Oyibo P, Jujjavarapu S, Meulah B, Agbana T, Braakman I, van Diepen A, et al. 2022; Schistoscope: an automated microscope with artificial intelligence for detection of Schistosoma haematobium eggs in resource-limited settings. Micromachines (Basel). 13:643. DOI: 10.3390/mi13050643. PMID: 35630110. PMCID: PMC9146062.

21. WHO. Diagnostic target product profiles for monitoring, evaluation and surveillance of schistosomiasis control programmes. Geneva, Switzerland: World Health Organization.
22. Bransky A, Larsson A, Aardal E, Ben-Yosef Y, Christenson RH. 2021; A novel approach to hematology testing at the point of care. J Appl Lab Med. 6:532–42. DOI: 10.1093/jalm/jfaa186. PMID: 33274357. PMCID: PMC7798949.

23. Ben-Yosef Y, Marom B, Hirshberg G, D'Souza C, Larsson A, Bransky A. 2016; The HemoScreen, a novel haematology analyser for the point of care. J Clin Pathol. 69:720–5. DOI: 10.1136/jclinpath-2015-203484. PMID: 26786235.

24. Fåhræus R, Lindqvist T. 1931; The viscosity of the blood in narrow capillary tubes. Am J Physiol. 96:562–8. DOI: 10.1152/ajplegacy.1931.96.3.562.

25. Leshansky AM, Bransky A, Korin N, Dinnar U. 2007; Tunable nonlinear viscoelastic "focusing" in a microfluidic device. Phys Rev Lett. 98:234501. DOI: 10.1103/PhysRevLett.98.234501. PMID: 17677908.

26. Bachar N, Benbassat D, Brailovsky D, Eshel Y, Glück D, Levner D, et al. 2021; An artificial intelligence-assisted diagnostic platform for rapid near-patient hematology. Am J Hematol. 96:1264–74. DOI: 10.1002/ajh.26295. PMID: 34264525. PMCID: PMC9290600.

27. An R, Man Y, Iram S, Kucukal E, Hasan MN, Huang Y, et al. 2021; Point-of-care microchip electrophoresis for integrated anemia and hemoglobin variant testing. Lab Chip. 21:3863–75. DOI: 10.1039/D1LC00371B. PMID: 34585199. PMCID: PMC9714341.

28. Soares Magalhães RJ, Clements AC. 2011; Spatial heterogeneity of haemoglobin concentration in preschool-age children in sub-Saharan Africa. Bull World Health Organ. 89:459–68. DOI: 10.2471/BLT.10.083568. PMID: 21673862. PMCID: PMC3099553.

Fig. 1
Schematic of the electrophoretic process for POC testing of anemia and Hb variants. (A) A whole blood specimen (red) is mixed with a standard calibrator (blue) and electrophoresed on the cellulose acetate paper. (B) Within 2.5 minutes, the Hb is separated from the standard. A ML ANN is used to determine the Hb concentration and anemia status. (C) Within 8 minutes, the Hb is separated into the different variants, and major variants are determined (Adapted from An, et al. [27].).
Abbreviations: POC, point-of-care; ML machine learning; ANN, artificial neural network.
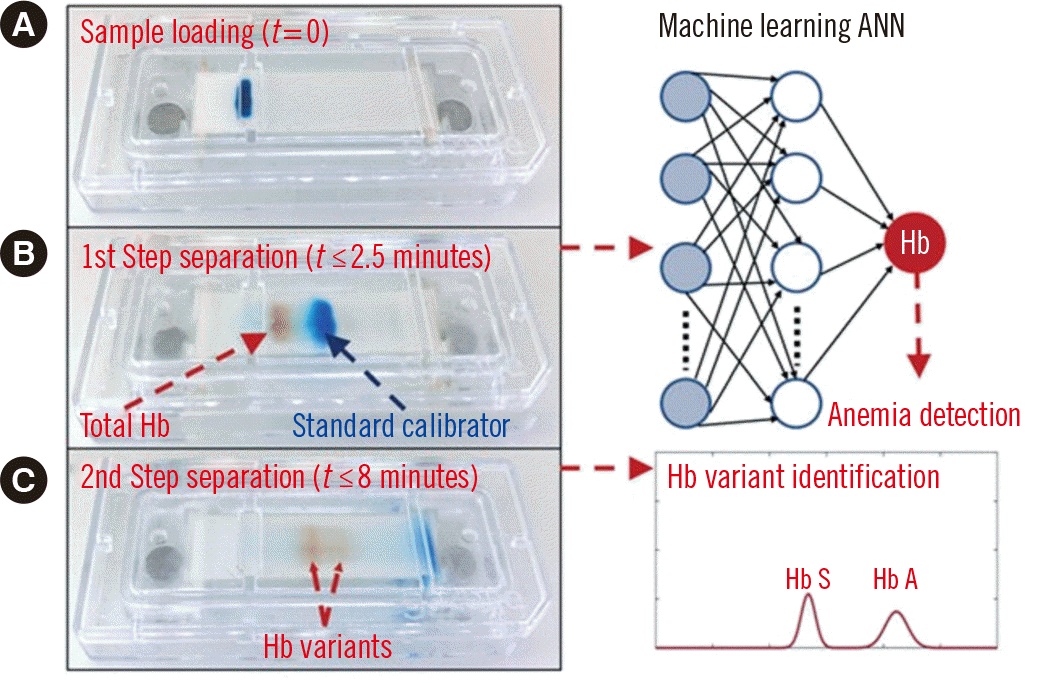
Table 1
Comparison of the features of the HemoScreen and Sight OLO hematology instruments, which use AI




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download