1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021; Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. DOI:
10.3322/caac.21660. PMID:
33538338.

2. Lim B, Kim JH, Kim M, Kim SY. 2016; Genomic and epigenomic heterogeneity in molecular subtypes of gastric cancer. World J Gastroenterol. 22:1190–1201. DOI:
10.3748/wjg.v22.i3.1190. PMID:
26811657. PMCID:
PMC4716030.

3. Ikeguchi M, Miyatani K, Takaya S, Matsunaga T, Fukumoto Y, Osaki T, Saito H, Wakatsuki T. 2016; Role of surgery in the management for gastric cancer with synchronous distant metastases. Indian J Surg Oncol. 7:32–36. DOI:
10.1007/s13193-015-0428-6. PMID:
27065679. PMCID:
PMC4811814.

5. Ma J, Hong L, Chen Z, Nie Y, Fan D. 2014; Epigenetic regulation of microRNAs in gastric cancer. Dig Dis Sci. 59:716–723. DOI:
10.1007/s10620-013-2939-8. PMID:
24248419.

7. Li M, Liu Y, Zhang X, Liu J, Wang P. 2018; Transcriptomic analysis of high-throughput sequencing about circRNA, lncRNA and mRNA in bladder cancer. Gene. 677:189–197. DOI:
10.1016/j.gene.2018.07.041. PMID:
30025927.

10. Tian T, Wang M, Lin S, Guo Y, Dai Z, Liu K, Yang P, Dai C, Zhu Y, Zheng Y, Xu P, Zhu W, Dai Z. 2018; The impact of lncRNA dysregulation on clinicopathology and survival of breast cancer: a systematic review and meta-analysis. Mol Ther Nucleic Acids. 12:359–369. DOI:
10.1016/j.omtn.2018.05.018. PMID:
30195774. PMCID:
PMC6037885.

11. Fattahi S, Kosari-Monfared M, Golpour M, Emami Z, Ghasemiyan M, Nouri M, Akhavan-Niaki H. 2020; LncRNAs as potential diagnostic and prognostic biomarkers in gastric cancer: a novel approach to personalized medicine. J Cell Physiol. 235:3189–3206. DOI:
10.1002/jcp.29260. PMID:
31595495.

12. López-Urrutia E, Bustamante Montes LP, Ladrón de Guevara Cervantes D, Pérez-Plasencia C, Campos-Parra AD. 2019; Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: deciphering molecular mechanisms of master regulators in cancer. Front Oncol. 9:669. DOI:
10.3389/fonc.2019.00669. PMID:
31404273. PMCID:
PMC6670781. PMID:
6987929dd3cf42c2afb8317153f61b43.

13. Karagkouni D, Paraskevopoulou MD, Tastsoglou S, Skoufos G, Karavangeli A, Pierros V, Zacharopoulou E, Hatzigeorgiou AG. 2020; DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 48:D101–D110. DOI:
10.1093/nar/gkz1036. PMID:
31732741. PMCID:
PMC7145509.

15. Xiao S, Guo J, Zhang W, Hu X, Wang R, Chen Z, Lai C. 2022; A six-microRNA signature nomogram for preoperative prediction of tumor deposits in colorectal cancer. Int J Gen Med. 15:675–687. DOI:
10.2147/IJGM.S346790. PMID:
35082517. PMCID:
PMC8785134.

16. Tang J, Hu Y, Zhang C, Gong C. 2021; miR-4636 inhibits tumor cell proliferation, migration and invasion, and serves as a candidate clinical biomarker for gastric cancer. Oncol Lett. 21:33. DOI:
10.3892/ol.2020.12294. PMID:
33262825. PMCID:
PMC7693299.

17. Yin S, Yang M, Li X, Zhang K, Tian J, Luo C, Bai R, Lu Y, Wang M. 2020; Peripheral blood circulating microRNA-4636/-143 for the prognosis of cervical cancer. J Cell Biochem. 121:596–608. DOI:
10.1002/jcb.29305. PMID:
31407404.

18. Duncan MC. 2022; New directions for the clathrin adaptor AP-1 in cell biology and human disease. Curr Opin Cell Biol. 76:102079. DOI:
10.1016/j.ceb.2022.102079. PMID:
35429729.

19. Toda H, Kurozumi S, Kijima Y, Idichi T, Shinden Y, Yamada Y, Arai T, Maemura K, Fujii T, Horiguchi J, Natsugoe S, Seki N. 2018; Molecular pathogenesis of triple-negative breast cancer based on microRNA expression signatures: antitumor miR-204-5p targets AP1S3. J Hum Genet. 63:1197–1210. DOI:
10.1038/s10038-018-0510-3. PMID:
30228364.

20. Ye T, Cheng Y, Li C. 2021; Adaptor protein complex 1 sigma 3 is highly expressed in glioma and could enhance its progression. Comput Math Methods Med. 2021:5086236. DOI:
10.1155/2021/5086236. PMID:
34367317. PMCID:
PMC8346305.

21. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. DOI:
10.1006/meth.2001.1262. PMID:
11846609.

22. Chen Q, Yao YT, Xu H, Chen YB, Gu M, Cai ZK, Wang Z. 2016; SPOCK1 promotes tumor growth and metastasis in human prostate cancer. Drug Des Devel Ther. 10:2311–2321. DOI:
10.2147/DDDT.S91321. PMID:
27486308. PMCID:
PMC4958368.
23. Zhou L, Zhu Y, Sun D, Zhang Q. 2020; Emerging roles of long non-coding RNAs in the tumor microenvironment. Int J Biol Sci. 16:2094–2103. DOI:
10.7150/ijbs.44420. PMID:
32549757. PMCID:
PMC7294937.

24. Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J, Rao X, Li M, Sun M, Jiang M, Xu Y, Teng L, Jin Y, Si J, Liu W, Kang Y, Zhou T. 2019; Long noncoding RNA GMAN, up-regulated in gastric cancer tissues, is associated with metastasis in patients and promotes translation of ephrin A1 by competitively binding GMAN-AS. Gastroenterology. 156:676–691.e11. DOI:
10.1053/j.gastro.2018.10.054. PMID:
30445010.

25. Yu Y, Li L, Zheng Z, Chen S, Chen E, Hu Y. 2017; Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J Cell Mol Med. 21:955–967. DOI:
10.1111/jcmm.13035. PMID:
27878953. PMCID:
PMC5387161.

26. Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY, Liu Q, Zhao G, Zhang ZZ. 2019; The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer. 18:115. Erratum in:
Mol Cancer. 2021;20:120. DOI:
10.1186/s12943-019-1032-0. PMID:
31272462. PMCID:
PMC6609402. PMID:
ee6fb104778948bc9014fe573397dfe8.

27. Idichi T, Seki N, Kurahara H, Fukuhisa H, Toda H, Shimonosono M, Okato A, Arai T, Kita Y, Mataki Y, Kijima Y, Maemura K, Natsugoe S. 2018; Molecular pathogenesis of pancreatic ductal adenocarcinoma: impact of passenger strand of pre-miR-148a on gene regulation. Cancer Sci. 109:2013–2026. DOI:
10.1111/cas.13610. PMID:
29660218. PMCID:
PMC5989856.

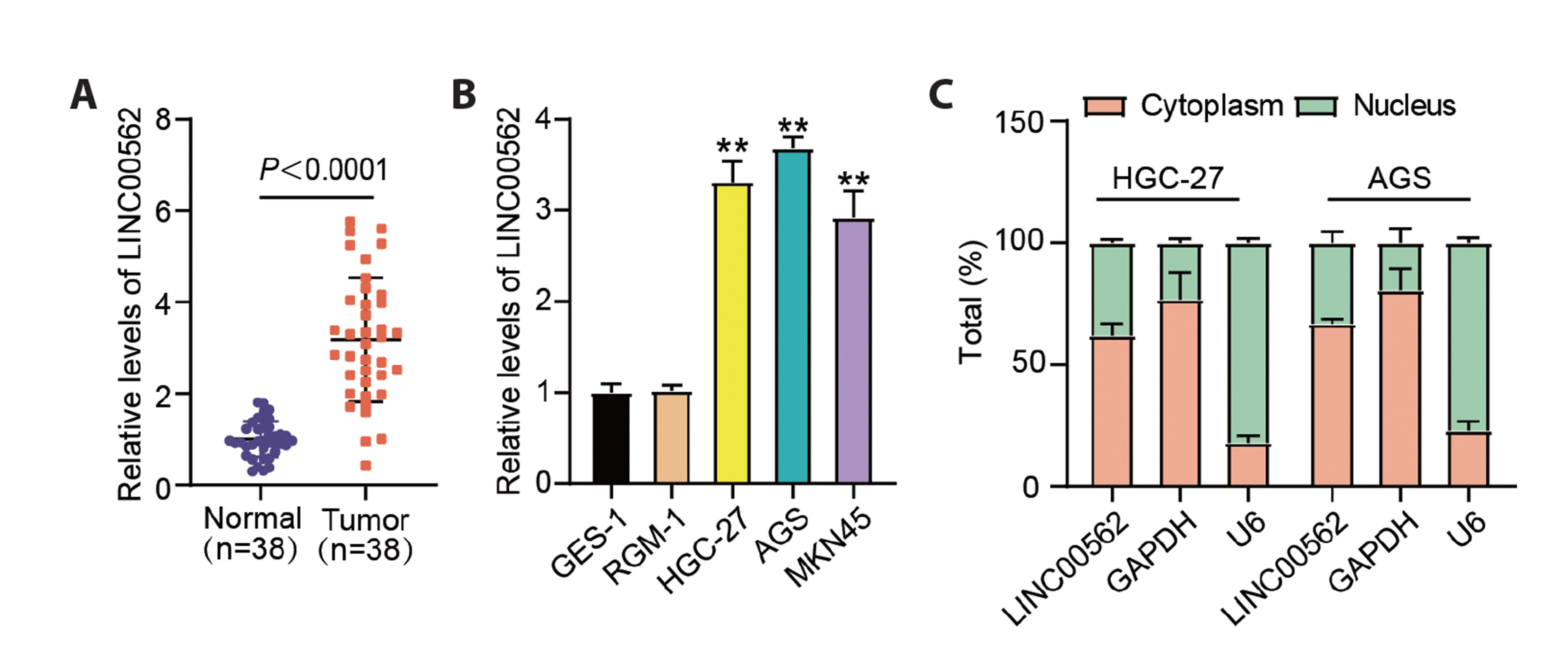

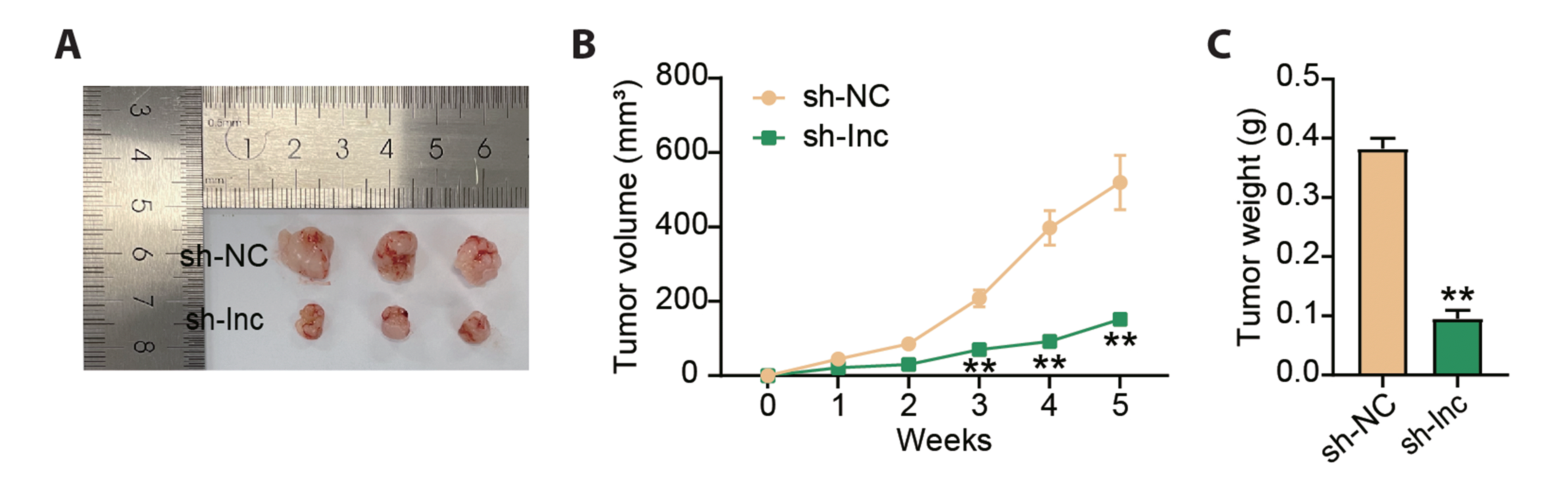






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download