This article has been corrected. See "Correction: Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool" in Volume 47 on page 228.
Abstract
Objective
To systematically translate the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool (DMDSAT) into Korean and verify the reliability and validity of the Korean version (K-DMDSAT).
Methods
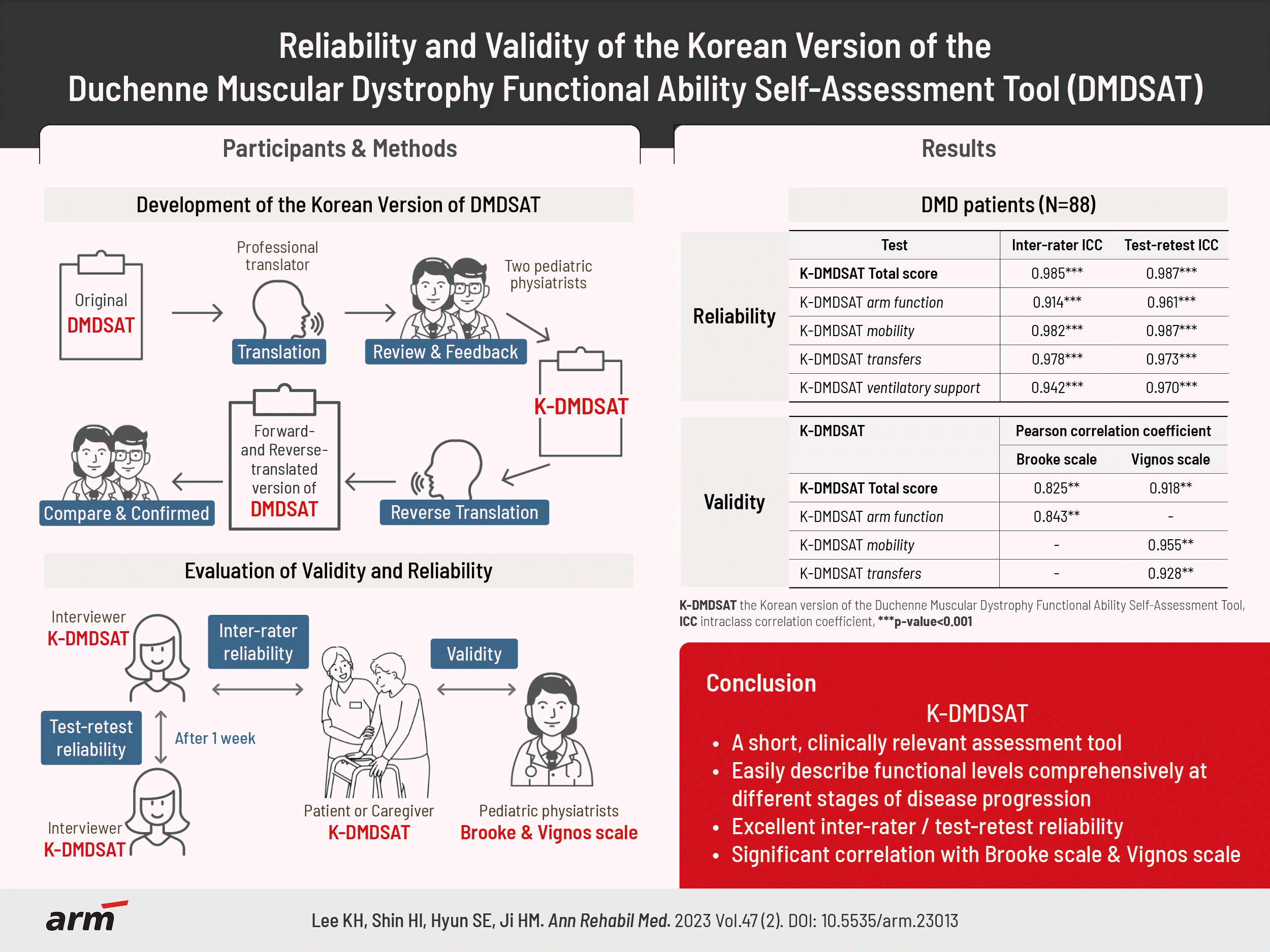
The original DMDSAT was translated into Korean by two translators and two pediatric physiatrists. A total of 88 patients with genetically confirmed Duchenne muscular dystrophy (DMD) participated in the study. They were evaluated using the K-DMDSAT once as a self-assessment and once by an interviewer. The interviewer evaluated the K-DMDSAT again 1 week later using a test-retest approach. The intraclass correlation coefficient (ICC) was used to verify the interrater and test-retest reliabilities. Pearson correlation analysis between the K-DMDSAT and the Brooke or Vignos scales were used to assess validity.
Results
The total score and all domains of the K-DMDSAT showed excellent interrater and test-retest reliability, with an ICC for total scores of 0.985 and 0.987, respectively. All domains had an ICC >0.90. From the Pearson correlation analysis, the total K-DMDSAT score was significantly correlated with the Vignos and Brooke scales (r=0.918 and 0.825, respectively; p<0.001), and each domain of K-DMDSAT showed significant correlation with either the Vignos or Brooke scales.
Duchenne muscular dystrophy (DMD) is the most common congenital muscular dystrophy, affecting approximately one in 5,000 live male births [1]. DMD is characterized by progressive muscle weakness and atrophy, mainly involving the proximal muscles, rather than the distal muscles. As DMD progresses, trunk muscles and respiratory muscles, including the diaphragm, also get affected; therefore, patients experience progressive decline not only in physical, but also in cardiac and respiratory function [2]. Since there is currently no curative treatment to stop disease progression yet [3], regular check-ups for various functional aspects of DMD, including muscle power; bony deformities, including scoliosis or hip dislocation; pain; and pulmonary and cardiac function, are essential for disease management and intervention [3-7]. However, because there is considerable heterogeneity in the rate of progression across patients, a reliable assessment tool that can describe the functional status of patients at each follow-up period in clinical practice is warranted.
The Duchenne muscular dystrophy Functional Ability Self-Assessment Tool (DMDSAT) is a patient-reported outcome scale developed to assess and describe the functional status of patients with DMD throughout the entire disease course [8]. It was created as an easy assessment tool for patients or caregivers and a clinically relevant tool. In a previous study, the English version of DMDSAT demonstrated good validity across the entire disease course and good reliability (Cronbach’s alpha, 0.93) [8]. Consequently, DMDSAT has been used to describe and categorize the functional ability of patients with DMD in the literature [4,9-11].
Furthermore, Landfeldt et al. [12,13] reported that DMD is associated with a considerable cost burden to society and affected families, and interventions for DMD based on DMDSAT were helpful in cost-effectiveness analysis [14]. Considering the worldwide prevalence of DMD [15], translating DMDSAT into languages other than English is necessary to facilitate its implementation. To the best of our knowledge, no study has translated DMDSAT or reported the validity of DMDSAT in a version other than English. Accordingly, this study aimed to systematically translate DMDSAT into Korean and verify the reliability and validity of the Korean version.
DMDSAT comprises four domains: arm function, mobility, transfers, and ventilatory support (Appendix 1). In the arm function and mobility domains, activities using the upper or lower limbs are listed in order of difficulty, and patients are asked to choose the one item that they feel is the most difficult task they can do. The original score ranges from 0 to 8 in both domains, with a higher score representing a more difficult activity. However, with the rescoring system [8], the score ranges from 0 to 6 in the arm function domain and from 0 to 5 in the mobility domain (Appendix 2).
In the transfers domain, patients are asked to choose how much assistance they require to complete each activity. If patients can perform an activity independently, 2 points are scored; if patients can do so with help, 1 point is scored; and if the patient cannot perform a certain activity or needs to be lifted or hoisted, no point is scored. With a sum of 5 items, the score ranges from 0 to 10 in the transfers domain.
In the ventilatory support domain, the patients are asked to choose one item that describes how much ventilatory support they required. Two points are given if they answered “not ventilated,” 1 point if they answer “ventilated at night,” and 0 point if they answered “ventilated during day and night.” Since the questionnaire is designed to assess the need for respiratory support in daily living, patients who are on ventilator day and night because of acute respiratory infections should not be included in the scoring system.
With the sum of scores of all four domains, the total score of the DMDSAT ranges between 0 and 23, with a higher score representing a higher functional activity or level of independence.
K-DMDSAT was developed by translating and reverse-translating the questionnaires. Before beginning the procedure, approval via e-mail was obtained from the original author and Newcastle University, the copyright-holders of DMDSAT. Forward translation was performed by a professional translator and reviewed by two Korean pediatric physiatrists fluent in both English and Korean (Appendix 3). Another translator performed reverse translation into English. The original version of DMDSAT and forward- and reverse-translated versions were compared; there were no conceptual inconsistencies between both versions.
Patients diagnosed with DMD who visited the pediatric outpatient clinic of our hospital between April 2020 and October 2021 were recruited for this study. DMD diagnosis was confirmed using a dystrophin gene study. To identify dystrophin mutations, multiplex polymerase chain reaction and direct sequencing (Xp21.2-p21.1, exons 1–79) were performed. If the deletion or duplication tests yielded negative results, dystrophin gene sequencing was performed to search for point mutations or small deletions or insertions. Written informed consent was obtained from all participants after explaining the purpose and content of this study. Patients or caregivers who could not fully understand the written questionnaires were excluded. A total of 88 patients agreed to participate, and none dropped out during the study. This study was conducted in accordance with the Declaration of the World Medical Association and was reviewed and approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-2106-062-1225).
This study was conducted to verify the interrater and test-retest reliability of K-DMDSAT. On the day of participation, patients were asked to complete the K-DMDSAT without assistance. However, in cases where the patient could not comprehend or understand the questions owing to a lack of reading ability, a proxy writer was allowed if he was living with parents or caregivers with whom he had a continuous relationship.
On the same day as the self-assessment, an interviewer asked questions on the DMDSAT items and recorded their responses to fill out the K-DMDSAT. The same interviewer once more evaluated the participants 1 week after the first evaluation. The K-DMDSAT score was measured, provided that participants did not experience any important medical or functional change in clinical status in a week. During the entire research period, the patient’s self-report of K-DMDSAT took approximately 10 minutes, and the interview took approximately 5 minutes. All assessments were performed at the outpatient clinic of the Department of Pediatric Rehabilitation Medicine.
The DMDSAT scores by self- and interviewer assessment were compared to verify interrater reliability, and results from the first and second assessments by the interviewer were compared to verify test-retest reliability. The intraclass correlation coefficient (ICC) was used for statistical analysis, where values between 0.74 and 1.0 represented excellent reliability, 0.60 and 0.74 represented good reliability, 0.40 and 0.59 represented fair reliability, and <0.4 represented poor reliability.
In this study, the Brooke scale [16] and Vignos scale [17], which are widely used functional classifications of patients with DMD for upper limb and mobility function, respectively, were used to verify the validity of K-DMDSAT. On the day of the first measurement for each participant, one pediatric physiatrist evaluated each patient’s function using the Brooke and Vignos scales. Since DMDSAT comprises various categories, Pearson correlation analysis between K-DMDSAT and the Brooke or Vignos scales were performed separately; the following combinations were analyzed: the total score of K-DMDSAT with the Brooke and Vignos scales, arm function domain of K-DMDSAT with the Brooke scale, mobility domain of K-DMDSAT with the Vignos scale, and transfers domain of K-DMDSAT with the Vignos scale. A p-value <0.05 was considered statistically significant. All analysis were performed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Table 1 shows the baseline characteristics of participants. The mean age of participants was 16.63±5.09 years, and two-thirds were nonambulant. Most participants did not use a ventilator at all, but 4 used a ventilator both day and night, and 7 used the ventilator only at night. Participants with various disease severity were included, ranging from 1 to 23 in the total K-DMDSAT score, with a median score of 9.
The interrater reliability of K-DMDSAT was significant for the total score and all 4 K-DMDSAT domains (Table 2). The ICC showed excellent reliability for all domains, including the total score; the ICC was 0.985 for the total score, 0.914 for the arm function domain, 0.982 for the mobility domain, 0.978 for the transfers domain, and 0.942 for the ventilatory support domain.
Similar results with interrater reliability were observed for the test-retest reliability (Table 3). K-DMDSAT showed significant and excellent reliability in the total score and all domains, with an ICC of 0.987 for the total score, 0.961 for the arm function domain, 0.987 for the mobility domain, 0.973 for the transfers domain, and 0.970 for the ventilatory support domain (p<0.001).
Pearson correlation analysis showed a significant correlation between the total score of the K-DMDSAT and the Vignos and Brooke scales (r=0.918 and 0.825, respectively, p<0.001). Specifically, the arm function domain was significantly correlated with the Brooke scale (r=0.843, p<0.001), and the mobility and transfers domains were significantly correlated with the Vignos scale (r=0.955 and r=0.928, respectively, p<0.001).
This study was designed to translate DMDSAT into Korean and investigate the reliability and validity of KDMDSAT. Results showed that K-DMDSAT had excellent interrater and test-retest reliability and validity, which supports using K-DMDSAT in clinical practice.
Similar results were reported by Landfeldt et al. [8], who developed the original DMDSAT. Among 186 patients from the United Kingdom, DMDSAT showed very good reliability, with a Cronbach’s alpha of 0.93. Moreover, the study demonstrated that a one-point increase in the total score of DMDSAT was significantly associated with a 5.3% change in per-patient annual cost. Although the present study did not investigate the association between K-DMDSAT and clinical outcomes or cost-effectiveness, to our knowledge, it was the first to show a significant association between K-DMDSAT and clinically widely used scales–the Brooke scale and Vignos scale.
Although K-DMDSAT is a patient-reported outcome scale, it shows excellent interrater reliability. There are several possible reasons for this finding. First, the questionnaires are simple and easy to understand since the measure is designed to be completed by patients or caregivers who are not health professionals. Moreover, the questionnaires were about daily activities performed every day, not specific tasks, meaning that the respondents could easily fill out the assessment tool without profoundly thinking. Second, the items for each domain are listed in order of difficulty, which helps recognize the patient’s current function. Finally, because the time to complete the assessment is less than 10 minutes, a more reliable response can be expected with low fatigue and higher compliance.
Since there is a consensus that motor function, which reflects activities of daily living, is more clinically relevant than conventional motor examination, such as manual muscle testing, for assessing patients with muscular dystrophy [18], several functional assessment tools for DMD or other types of muscular dystrophy have been developed. For example, the Brooke scale [16] and Vignos scale [17] were developed to assess each body part, upper and lower limbs, respectively. The North Star Ambulatory Assessment [19], motor function measure [20], and ambulatory functional classification system for DMD [21] were developed to document the ambulatory or motor function of the patient. These scales have demonstrated significant reliability and validity in previous studies. However, since each instrument is related to only one functional aspect, two or more assessment tools should be utilized to comprehensively measure and document the functional status of patients with DMD. Conversely, K-DMDSAT concerns four functional aspects simultaneously, so it appears to be an appropriate tool for quickly identifying and documenting overall functional status.
The Comprehensive Functional Scale for DMD (CFSD) [21], developed as a simplified tool to assess the functional abilities of patients with DMD, includes not only body structures such as joint contracture or Cobb’s angle for scoliosis but also intellectual disability, participation at school or society, and the ability to use personal computers or powered wheelchairs, which reflect the utilization of technology. However, CFSD comprises 21 items in three categories and takes more time to complete owing to several factors, including checking the range of motion, calculating Cobb’s angle, and testing intellectual disability, making it difficult to use in clinical settings and only suitable for research purposes.
The Egen Klassifikation (EK) scale was also designed for the same purpose and extended in 2008 to include more functional abilities, including swallowing function (e.g., food textures) and distal hand function (e.g., ability to control joystick) [22]. However, since it was developed for the functional assessment of nonambulatory patients, there are some limitations in its application in all patients with DMD.
Compared to these two well-validated comprehensive scales, the CFSD and EK scales, the DMDSAT lacks items assessing other aspects of DMD, especially dysphagia and pain. Since dysphagia and pain are symptoms that require careful attention and are strongly associated with quality of life [23], extending to DMDSAT questionnaires on swallowing function and pain would provide more information and help plan the most appropriate intervention. Although DMDSAT is a scoring system, its purpose is to document functional status rather than to provide a quantitative evaluation of physical function. Therefore, we propose adding items on dysphagia and pain to the original format.
In addition, we believe a supplementary item should be considered for the scoring system of the ventilatory support domain of DMDSAT. Most patients typically begin ventilator use at night and eventually increase use during the day if there is dyspnea during the day or elevated daytime carbon dioxide levels despite adequate night treatment [24]. However, variability in ventilator use in DMD patients exists depending on the patient’s comfort and compliance with mechanical ventilation. In fact, some patients use only mouthpiece ventilation during the day, whereas others prefer nasal interfaces for ventilator support and alternate them throughout the day [25]. Additionally, rarely, some patients prefer ventilator support only when they are awake, so they don’t use the ventilator during the night [26]. According to the current DMDSAT items, it is difficult to evaluate such patients because they are not included in any given categories. Although, fortunately, there were no such patients in this study, there may be difficulty in evaluating DMDSAT scores for such patients in future clinical settings. Therefore, we propose assigning 1 point for patients who use ventilatory support only during the daytime.
This study had several limitations. Among the participants, only 11 (12.5%) patients received ventilator support. The mean score of each K-DMDSAT domain for this population was 1.63±1.29 for the arm function domain, 0.27±0.46 for the mobility domain, and 0 for the transfer domain. There might have been a bottom effect, though it was not investigated in the current study. Since there has been considerable improvement in the life span of patients with DMD owing to advances in standards of care, especially in respiratory management [27-29], additional items that could describe the functional status of ventilator users are needed. Moreover, the K-DMDSAT was not designed to consider children’s functional development, meaning that there is no age-specific information or score modification according to the patient’s age. Further studies to investigate the validity of K-DMDSAT in younger patients are warranted.
In conclusion, DMDSAT was systematically translated into Korean, and K-DMDSAT was verified to have excellent reliability and validity. K-DMDSAT is a short, clinically relevant assessment tool for patients with DMD that can be rapidly administered without assistantance. K-DMDSAT can help clinicians easily describe functional levels comprehensively at different stages of disease progression.
Notes
Conceptualization: Ji HM, Shin HI. Methodology: Ji HM, Hyun SE, Shin HI. Formal analysis: Lee K, Ji HM. Project administration: Lee K, Ji HM. Visualization: Lee K. Writing – original draft: Lee K. Writing – review and editing: Lee K, Shin HI, Hyun SE, Ji HM. Approval of final manuscript: all authors.
REFERENCES
1. Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014; 24:482–91.

2. Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987; 51:919–28.

3. Mah JK. An overview of recent therapeutics advances for Duchenne muscular dystrophy. Methods Mol Biol. 2018; 1687:3–17.

4. Kim A, Park M, Shin HI. Pain characteristics among individuals with Duchenne muscular dystrophy according to their clinical stage. BMC Musculoskelet Disord. 2022; 23:536.

5. Canavese F, Sussman MD. Strategies of hip management in neuromuscular disorders: Duchenne Muscular Dystrophy, Spinal Muscular Atrophy, CharcotMarie-Tooth Disease and Arthrogryposis Multiplex Congenita. Hip Int. 2009; 19 Suppl 6:S46–52.

6. Mayer OH, Finkel RS, Rummey C, Benton MJ, Glanzman AM, Flickinger J, et al. Characterization of pulmonary function in Duchenne Muscular Dystrophy. Pediatr Pulmonol. 2015; 50:487–94.

7. Buddhe S, Cripe L, Friedland-Little J, Kertesz N, Eghtesady P, Finder J, et al. Cardiac management of the patient with Duchenne Muscular Dystrophy. Pediatrics. 2018; 142(Suppl 2):S72–81.

8. Landfeldt E, Mayhew A, Eagle M, Lindgren P, Bell CF, Guglieri M, et al. Development and psychometric analysis of the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool (DMDSAT). Neuromuscul Disord 2015;25:937-44. Erratum in: Neuromuscul Disord. 2016; 26:329.

9. El-Aloul B, Speechley KN, Wei Y, Wilk P, Campbell C. Fatigue in young people with Duchenne muscular dystrophy. Dev Med Child Neurol. 2020; 62:245–51.

10. Arteaga D, Donnelly T, Crum K, Markham L, Killian M, Burnette WB, et al. Assessing physical activity using accelerometers in youth with Duchenne muscular dystrophy. J Neuromuscul Dis. 2020; 7:331–42.

11. Fujii T, Takeshita E, Iwata Y, Yajima H, Nozaki F, Mori M, et al. Cumulative jerk as an outcome measure in nonambulatory Duchenne muscular dystrophy. Brain Dev. 2019; 41:796–802.

12. Landfeldt E, Lindgren P, Bell CF, Guglieri M, Straub V, Lochmüller H, et al. Quantifying the burden of caregiving in Duchenne muscular dystrophy. J Neurol. 2016; 263:906–15.

13. Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014; 83:529–36.

14. Landfeldt E, Alfredsson L, Straub V, Lochmüller H, Bushby K, Lindgren P. Economic evaluation in Duchenne muscular dystrophy: model frameworks for cost-effectiveness analysis. Pharmacoeconomics. 2017; 35:249–58.

15. Salari N, Fatahi B, Valipour E, Kazeminia M, Fatahian R, Kiaei A, et al. Global prevalence of Duchenne and Becker muscular dystrophy: a systematic review and meta-analysis. J Orthop Surg Res. 2022; 17:96.

16. Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981; 4:186–97.

17. Vignos PJ Jr, Archibald KC. Maintenance of ambulation in childhood muscular dystrophy. J Chronic Dis. 1960; 12:273–90.

18. Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond). 2011; 1:1217–35.

19. Mazzone ES, Messina S, Vasco G, Main M, Eagle M, D’Amico A, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009; 19:458–61.

20. Bérard C, Payan C, Hodgkinson I, Fermanian J; MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005; 15:463–70.
21. Kim J, Jung IY, Kim SJ, Lee JY, Park SK, Shin HI, et al. A new functional scale and ambulatory functional classification of Duchenne muscular dystrophy: scale development and preliminary analyses of reliability and validity. Ann Rehabil Med. 2018; 42:690–701.

22. Mayhew AG, Eagle M, Steffenson B. S.P.6 Exploratory Rasch analysis of the EK2 scale used in a population of Duchenne muscular dystrophy (DMD). Neuromuscul Disord. 2012; 22:877.

23. Huang M, Chen T, Wang Y, Zhou C, Cao J, Lu X, et al. Chronic pain, psychological distress, and quality of life in males with Duchenne muscular dystrophy. Dev Med Child Neurol. 2023; 65:640–54.

24. MacKintosh EW, Chen ML, Benditt JO. Lifetime care of Duchenne muscular dystrophy. Sleep Med Clin. 2020; 15:485–95.

25. Fiorentino G, Annunziata A, Cauteruccio R, Frega GS, Esquinas A. Mouthpiece ventilation in Duchenne muscular dystrophy: a rescue strategy for noncompliant patients. J Bras Pneumol. 2016; 42:453–6.

26. Hurvitz MS, Bhattacharjee R, Lesser DJ, Skalsky AJ, Orr JE. Determinants of usage and nonadherence to noninvasive ventilation in children and adults with Duchenne muscular dystrophy. J Clin Sleep Med. 2021; 17:1973–80.

27. Gomez-Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. Am J Phys Med Rehabil. 2002; 81:411–5.
Table 1.
Clinical characteristics of participants
Table 2.
Interrater reliability of the K-DMDSAT (n=88)
| K-DMDSAT | Self | Interviewer | ICC |
|---|---|---|---|
| Total score | 11.60±7.16 | 11.73±7.40 | 0.985*** |
| Arm function | 4.65±1.69 | 4.67±1.61 | 0.914*** |
| Mobility | 1.82±1.78 | 1.82±1.86 | 0.982*** |
| Transfers | 3.49±4.16 | 3.44±4.29 | 0.978*** |
| Ventilatory support | 1.83±0.49 | 1.80±0.53 | 0.942*** |
Appendices
Appendix 1.
Original version of the Duchenne muscular dystrophy (DMD) Functional Ability Self-Assessment Tool
The DMD Functional Ability Self-Assessment Tool (DMDSAT)
The questions below describe the levels of activity for arm function, mobility, transfers, and need for ventilatory support. The activities are intended to be in order of difficulty, and we would like you to tick the box that best applies to your current level of function.
| Ventilatory support | Not ventilated | Ventilated at night | Ventilated during day and night |
|---|---|---|---|
| Ventilatory status | ● | ● | ● |
Appendix 2.
Re-scoring system of the arm function and mobility domain of the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool
Appendix 3.
Korean version of the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool
뒤셴 근이영양증 자가 기능 검사
아래는 팔 기능, 보행 및 자세 변환의 기능 수준과 폐 기능을 파악하는 설문입니다. 활동은 쉬운 동작에서 어려운 동작 순으로 나열되어 있으니 본인의 현재 상태에 가장 적합한 선택지를 표기해 주십시오.
| 이동 | 스스로 할 수 있음 | 도움이 필요함 | 견인이 필요하거나 불가능함 |
|---|---|---|---|
| 바닥에서 일어나기/바닥에 앉기 | ● | ● | ● |
| 의자에서 일어나기/의자에 앉기 | ● | ● | ● |
| 침대 오르내리기 | ● | ● | ● |
| 변기에서 일어나기/변기에 앉기 | ● | ● | ● |
| 계단 오르내리기 | ● | ● | ● |
| 호흡 보조 | 호흡 보조 장치를 사용하지 않음 | 밤에 호흡 보조 장치를 사용함 | 낮과 밤 모두 호흡 보조 장치를 사용함 |
|---|---|---|---|
| 호흡 상태 | ● | ● | ● |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download