This article has been
cited by other articles in ScienceCentral.
Abstract
Neurolymphomatosis is the direct endoneurial infiltration of lymphoma cells. Bone marrow biopsy is a widely practiced procedure that is generally considered to be relatively safe. However, bone marrow biopsy can also result in pain and long-term consequences such as nerve injury. Here we report a case of a 68-year-old male who presented with lumbosacral plexopathy due to neurolymphomatosis that was superimposed on a probable traumatic lumbosacral plexopathy mostly involving the sciatic nerve immediately after a bone marrow biopsy.
Keywords: Neurolymphomatosis, Peripheral nerve injuries, Bone marrow, Biopsy
Bone marrow biopsy is a widely practiced procedure that is considered to be relatively safe. However, it can cause pain and long-term consequences such as nerve injury. There have been a few case reports of mechanical nerve root injury or compressive neuropathy due to a hematoma or gluteal artery pseudoaneurysm.
1-
3
Neurolymphomatosis is the direct endoneurial infiltration of lymphoma cells. Nervous system invasion occurs in approximately 5% of patients with lymphoma, especially in those with diffuse large-B-cell lymphoma (DLBCL).
4 Here we report the first case of lumbosacral plexopathy due to neurolymphomatosis that was superimposed on a probable traumatic lumbosacral plexopathy mostly involving the sciatic nerve immediately after a bone marrow biopsy.
CASE
A 68-year-old male presented with severe pain in his left posterior thigh immediately after a bone marrow biopsy. He had been diagnosed with stage IV DLBCL 5 months previously, for which he had undergone chemotherapy. In order to evaluate the response to chemotherapy, a bone marrow biopsy was performed in the left sacral area around the posterior iliac crest, with the patient lying in the prone position under local anesthesia. The procedure took about 30 minutes. Fluorine-18-fluorodeoxyglucose positron-emission tomography (
18F-FDG PET) was performed on the same day, which only revealed residual reactive lymph nodes in the mediastinum and bilateral pulmonary hilum (
Fig. 1A). Immediately after the biopsy, the patient complained of severe pain in the left posterior thigh (score of 10 on a numeric rating scale from 1 to 10) that radiated from his left buttock to his left sole, with no definite motor weakness. He also reported hypesthesia in the left posterior thigh.
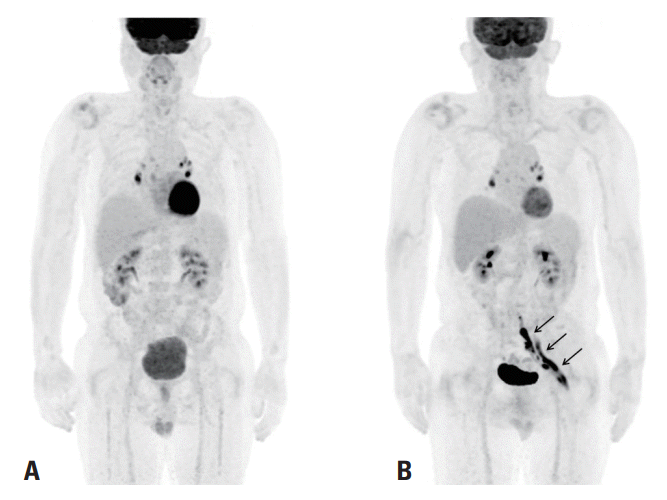
Fig. 1.
(A) Fluorine-18-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) on the day of bone marrow biopsy revealed residual reactive lymph nodes only in the mediastinum and bilateral pulmonary hilum (B). 18F-FDG PET revealed newly presenting hypermetabolism at the left lumbosacral plexus and sciatic nerve (straight arrows) at 6 weeks after the initial development of left leg weakness.

He underwent a nerve conduction study (NCS) and needle electromyography 2 months later. Sensory nerve action potential (SNAP) amplitudes were decreased on the left-side superficial peroneal and sural nerves. Although the compound muscle action potentials (CMAPs) of the tibial nerves were symmetrical, the CMAP amplitudes were slightly decreased in the left-side peroneal nerve when recorded at the tibialis anterior muscles, and absent in that nerve when recorded at the left extensor digitorum brevis. The left vastus lateralis, tibialis anterior, and medial gastrocnemius muscles had high-amplitude motor unit action potentials (MUAPs) on needle electromyography, but the interference pattern was complete. Abnormal spontaneous activity did not present in any of the muscles. Based on clinical symptoms including hypesthesia in the left posterior thigh, the study revealed lumbosacral plexopathy mostly involving the sciatic nerve with mild partial axonal loss, which was clinically comparable to probable traumatic neuropathy related to the bone marrow biopsy. Computed tomography of the abdomen and pelvis performed 2 weeks after the electrodiagnostic study revealed no definite lesions such as hematoma or aneurysm in the pelvis.
The patient revisited the clinic 1 month later with newly developed, rapidly progressing left leg weakness that first appeared after the initial examination. A neurological examination revealed motor weakness of grades 1-4 on the Medical Research Council scale in the left lower extremity: left hip flexor, grade 4; hip extensor, grade 3; knee flexor, grade 2; knee extensor, grade 3; ankle dorsiflexor, grade 1; ankle plantar flexor, grade 1; and long toe extensor, grade 1. The patient was unable to walk without assistance within 1 month. The NCS was performed the day after visiting the clinic (
Table 1). The SNAP amplitudes in the left superficial peroneal and sural nerves were severely decreased compared with those on the right side. The amplitudes of CMAP in the peroneal nerve recorded from the left tibialis anterior and left tibial nerve were severely decreased compared with those on the right side. The CMAP was absent in the peroneal nerve when recorded from the left extensor digitorum brevis. No F-waves were observed in the left extensor digitorum brevis and abductor hallucis muscles. Needle electromyography revealed prominent active denervation potentials in the left tibialis anterior, medial head of the gastrocnemius, abductor hallucis, peroneus longus, long and short heads of the biceps femoris, gluteus medius, and gluteus maximus muscles, which were not observed in the previous study (
Table 2). There were no MUAPs in the above muscles except for the left gluteus medius, which revealed a discrete interference pattern. The electrodiagnostic study demonstrated acute left lower lumbosacral plexopathy that mostly involved the sciatic and gluteal nerves, with moderate-to-severe partial axonal involvement.
Table 1.
Nerve conduction study findings conducted 1 month after developing left leg weakness
|
Nerve (recorded structure) |
Stimulation site |
Latency (ms)
|
Amplitude
|
Conduction velocity (m/s)
|
|
Right |
Left |
Right |
Left |
Right |
Left |
|
Motor NCS |
|
|
|
(mV) |
(mV) |
|
|
|
Common peroneal (EDB) |
Ankle |
4.08 |
NR*
|
3.3 |
NR*
|
|
|
|
Fibular head |
11.15 |
NR*
|
2.5 |
NR*
|
45.3 |
|
|
Common peroneal (TA) |
Fibular head |
3.33 |
5.23 |
6.3 |
0.7*
|
|
|
|
Tibial (AH) |
Ankle |
3.81 |
5.21 |
11.1 |
0.5*
|
|
|
|
Popliteal fossa |
11.38 |
14.46 |
7.7 |
0.4*
|
45.0 |
36.8*
|
|
Femoral (VM) |
Anterior thigh |
5.71 |
6.02 |
7.6 |
6.2 |
|
|
|
F-wave |
|
|
|
|
|
|
|
|
Common peroneal (EDB) |
Ankle |
50.9 |
NR*
|
|
|
|
|
|
Tibial (AH) |
Ankle |
50.9 |
NR*
|
|
|
|
|
|
H-reflex |
|
|
|
|
|
|
|
|
Tibial (soleus) |
Popliteal fossa |
NR*
|
NR*
|
|
|
|
|
|
Sensory NCS |
|
|
|
(µV) |
(μV) |
|
|
|
Superficial peroneal (foot dorsum) |
Lower leg |
2.50 |
2.25 |
18.2 |
2.6*
|
|
|
|
Sural (lateral malleolus) |
Calf |
2.38 |
2.42 |
22.1 |
2.5*
|
|
|
|
Saphenous (medial malleolus) |
Lower leg |
1.85 |
2.27 |
7.2 |
8.6 |
|
|
|
LFC (thigh) |
Inguinal ligament |
2.25 |
2.63 |
7.9 |
6.6 |
|
|

Table 2.
Needle electromyography findings conducted 1 month after developing left leg weakness
|
Muscle (left) |
ASA
|
MUAP
|
Interference pattern |
|
Fib/PSW |
Others |
Amplitude |
Duration |
Polyphasicity |
|
Tibialis anterior |
3+/3+ |
None |
|
|
|
No activity |
|
Medial gastrocnemius |
3+/3+ |
None |
|
|
|
No activity |
|
Abductor hallucis |
2+/2+ |
None |
|
|
|
No activity |
|
Vastus medialis |
None |
None |
Normal |
Normal |
Normal |
Discrete |
|
Adductor magnus |
None |
None |
Normal |
Normal |
Normal |
Reduced |
|
Peroneus longus |
3+/3+ |
None |
|
|
|
No activity |
|
Biceps femoris (long head) |
2+/2+ |
None |
|
|
|
No activity |
|
Biceps femoris (short head) |
2+/2+ |
None |
|
|
|
No activity |
|
Gluteus maximus |
2+/2+ |
None |
|
|
|
No activity |
|
Gluteus medius |
2+/2+ |
None |
Normal |
Normal |
Normal |
Discrete |
|
Iliopsoas |
None |
None |
Normal |
Normal |
Normal |
Reduced |
|
L5 paraspinal |
None |
None |
|
|
|
|

The follow-up
18F-FDG PET revealed a fusiform FDG-avid mass at the left lumbosacral plexus from the lumbosacral nerve roots to the sciatic nerve, which was compatible with neurolymphomatosis (
Fig. 1B). Contrast-enhanced magnetic resonance imaging (MRI) of the pelvis demonstrated markedly enlarged left S1 and S2 nerves with fascicular thickening and intense contrast enhancement, which proximally extended along the S1 nerve at the cauda equina and distally extended to the sciatic nerve, indicating neurolymphomatosis (
Supplementary Fig. 1).
After admission to the hemato-oncology department, the patient received a high dose of intravenous methotrexate and chemotherapy with intrathecal methotrexate. At the 6-month follow-up, the electrodiagnostic study revealed that the left lower lumbosacral plexopathy was aggravated.
DISCUSSION
Bone marrow biopsy is a relatively safe procedure with a low complication rate. However, there have been a few case reports of nerve injury after a bone marrow biopsy.
1-
3 The current case presented severe pain immediately after a bone marrow biopsy. The initial electrodiagnostic study did not reveal any abnormal spontaneous activity, suggesting that there were no ongoing nerve injuries. The initial
18F-FDG PET also did not reveal any abnormalities in the lumbosacral plexus. This neuropathic pain was therefore assumed to be a mild and direct injury to the lumbosacral plexus that mostly involved the sciatic nerve and was related to the biopsy procedure.
The patient developed left lower extremity weakness 2 months after the bone marrow biopsy. At that point, clinical suspicions of other etiologies appeared. The subsequent electrodiagnostic study revealed acute left lower lumbosacral plexopathy with prominent active denervation potentials, which had not appeared in the first study. Furthermore, 18F-FDG PET and pelvic MRI revealed lesions at the lumbar plexus, which were compatible with neurolymphomatosis.
Needle-tract seeding is extremely rare in bone marrow biopsy for non-Hodgkin lymphoma. We could only find three case reports of needle tract seeding after bone marrow biopsy in patients with non-Hodgkin lymphoma.
5-
7 Subcutaneous seeding occurred along the needle tract in those previous studies. In the current case, the follow-up
18F-FDG PET revealed a fusiform FDG-avid mass at the left lumbosacral plexus and no metastasis in subcutaneous tissues. Probable traumatic sciatic nerve injury and neurolymphomatosis therefore seemed to have occurred independently.
Comprehensive consideration of the clinical information, imaging studies, tissue biopsy, and cerebrospinal fluid analysis is needed for neurolymphomatosis diagnosis.
8 Neurolymphomatosis clinically presents as painful polyneuropathy or polyradiculopathy, which occurred in 31% of patients in a previous case series as in the present case, followed by painless polyneuropathy in 28%, cranial neuropathy in 21%, and mononeuropathy in 15%.
9 Although nerve biopsy is still the gold standard for neurolymphomatosis diagnosis, it is difficult to perform and can result in permanent nerve injury. Noninvasive imaging studies therefore have played increasingly important roles in prompt and accurate neurolymphomatosis diagnosis. Neurolymphomatosis generally presents in
18F-FDG PET as a linear or fusiform FDG-avid mass along a nerve pathway, and presents on MRI as diffuse enlargement of the nerves with hyperintensity on T2-weighted images and contrast enhancement.
10 Neurolymphomatosis has a poor prognosis, with a median overall survival time of patients with neurolymphomatosis of 10 months from the initial diagnosis.
8 Most patients with neurolymphomatosis undergo systemic chemotherapy alone, combined intrathecal chemotherapy, or external beam radiotherapy.
8
This is the first case report in the Republic of Korea of traumatic sciatic neuropathy and neurolymphomatosis occurring in the same patient. Although neurolymphomatosis is rare, lumbosacral plexopathy due to neurolymphomatosis can be superimposed on a traumatic nerve injury during bone marrow biopsy, and both conditions can be misdiagnosed. Clinical suspicion and accurate diagnosis using imaging and electrodiagnostic techniques are therefore needed for prompt and appropriate treatment, such as chemotherapy.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download