Abstract
Acute pulmonary embolism (PE) is a life-threatening disease that manifests with cardiorespiratory symptoms. Syncope can be a rare, but warning sign of PE. We report a case of a 49-year-old male diagnosed with PE who presented with recurrent syncope prior to typical cardiorespiratory symptoms. His computed tomography pulmonary angiogram revealed bilateral PE. Syncope can be a rare clinical symptom of PE, but considering lethality of the disease, a differential diagnosis of PE should be considered in patients with recurrent syncope.
Pulmonary embolism (PE) is caused by a blockage of pulmonary arteries by blood clotting, and is frequently related to deep vein thrombosis (DVT). PE is one of the most common causes of cardiovascular death. However, its early diagnosis can lead to appropriate treatment and hence reduced mortality.1 PE typically presents with cardiorespiratory symptoms including dyspnea, cough, pleuritic chest pain, tachypnea, and hypotension.1 Syncope is a clinical condition of transient consciousness loss caused by temporary cerebral hypoperfusion, and its cardiovascular origin must be differentiated. Here we report a rare PE case that presented with recurrent syncope.
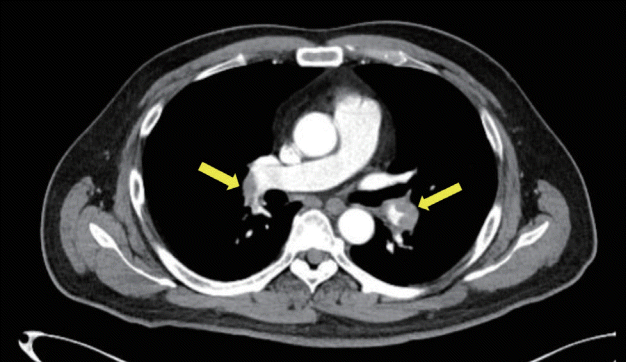
A 49-year-old male visited an emergency room (ER) due to recurrent syncope. He had a splint on his left ankle due to fracturing the navicular bone 1 month prior to his visit. He was diagnosed with diabetes mellitus and hypertension. He was taking valsartan, glimepiride, and metformin. He lost consciousness for less than 1 minute followed by blurred vision and dizziness. He had no history of specific triggers or epileptic features. He initially had no cardiovascular symptoms such as palpitation, dyspnea, or chest pain. His neurological examination produced no remarkable findings. His initial blood pressure was 95/69 mmHg heart rate was 109 beats/minute. His heart rate showed borderline elevation (131 beats/minute) without a decrease in blood pressure in the standing position. His electrocardiogram (ECG) revealed sinus tachycardia with no other specific abnormalities. Routine laboratory test results were within the normal limits, except for elevated D-dimer (8.69 mg/mL; normal range 0-0.5 mg/mL). Nonenhanced brain computed tomography (CT) produced no abnormal finding. He was initially discharged from the ER, but syncope recurred 3 hours later, and he revisited our ER. At his second visit he complained of mild atypical chest pain and dyspnea. Tenderness on left calf was observed in an additional physical examination. To differentiate pulmonary thromboembolism, CT pulmonary angiogram was performed, which identified bilateral massive PE (Fig. 1). A lower extremity ultrasonogram revealed DVT in the left popliteal and soleal veins. He was admitted and then treated using subcutaneous injection of low-molecular-weighted heparin at 80 mg twice per day for 1 week, followed by oral administration of rivaroxaban at 15 mg twice daily until the last follow-up. His clinical symptoms were improved after anticoagulation.
PE can present with various clinical symptoms, ranging from asymptomatic to severe dyspnea or chest pain that notoriously mimics other common medical conditions, and hence it can be easily underrecognized. The literature describes that routine cardiopulmonary tests such as ECGs, cardiac enzymes, and chest X-rays may produce normal findings in many cases.2 These clinical and laboratory features make this disease challenging to diagnose.
Hemodynamic instability is rarely seen in PE, which leads to persistent low systolic blood pressure (<90 mmHg, for more than 15 minutes), and it can be a warning sign of patients with massive PE.1 Only a small proportion of PE cases (5%) present with clinical symptoms related to hemodynamic instability, but PE with hemodynamic instability has a high mortality rate that exceeds 15%.2 In accordance with clinical symptoms related to hemodynamic instability, syncope can be the symptom that presents. One possible pathophysiology of PE-induced syncope is reduced cardiac output after failure of the right ventricle (RV) and impairment of left-ventricle filling, which is caused by occlusion in the pulmonary vessels. RV-strain-induced arrhythmia and vasovagal reflex induced by embolism have also been suggested as mechanisms underlying PE-induced syncope.3 This is known to be associated with RV dysfunction, which leads to a high risk of PE-related adverse events.4
Syncope can be classified by neurally mediated syncope, orthostatic hypotension, and cardiovascular origin. Compared with neurally mediated syncope and orthostatic hypotension, cardiovascular-origin syncope can lead to a life-threatening disease course. Among them, PE within the cardiovascular origin can have a fatal prognosis, but it is rarely considered as a cause of syncope in clinical analyses.5,6 The literature suggests that the prevalence of PE is lower than 1% among all patients who visit ERs with syncope.7 In accordance with previous studies, although PE-induced syncope is rare, it is critical to include this disease entity in the differential diagnosis of syncope due to its possible fatal outcome.4,7 In clinical practice, the diagnostic workup for cardiovascular syncope focuses on differentiating between arrhythmia and ischemic heart disease, and rarely considers the possibility of PE. In real-world data, PE rarely presents with isolated syncope without classical cardiorespiratory symptoms, which is frequently underrecognized.3,6
The pretest Wells’ score is a commonly used scoring system that can be easily implemented on a clinical basis, and the possibility of PE can be ruled out with scores greater than 4 (Table 1).8 In our case, the patient scored 6 on this scale, and we should therefore have suspected the presence of PE. PE should therefore be considered as a possible cause of recurrent syncope attacks. Our case suggested the clinical manifestation of recurrent syncope in PE and may improve the awareness among clinicians of these atypical findings, which could eventually lead to early diagnosis and prompt treatment.
REFERENCES
1. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019; 54:1901647.
2. Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020; 370:m2177.

3. Suwanwongse K, Shabarek N. Recurrent syncope as a presentation of pulmonary embolism. Cureus. 2020; 12:e6623.

4. Barco S, Ende-Verhaar YM, Becattini C, Jimenez D, Lankeit M, Huisman MV, et al. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2018; 39:4186–4195.

5. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018; 39:1883–1948.

6. Prandoni P, Lensing AW, Prins MH, Ciammaichella M, Perlati M, Mumoli N, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016; 375:1524–1531.

7. Costantino G, Ruwald MH, Quinn J, Camargo CA Jr, Dalgaard F, Gislason G, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018; 178:356–362.

8. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001; 135:98–107.

Fig. 1.
Computed tomography angiography of the patient. This image shows bilateral pulmonary embolism (arrows).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download