Abstract
Background
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
Methods
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
The global coronavirus disease 2019 (COVID-19) pandemic has also changed the epidemiology of non-infectious diseases [1]. Moreover, the decrease in physical activity due to social distancing has led to a sharp increase in the overall prevalence of overweight and obesity [2]. Isolation to prevent the spread of COVID-19, or drugs such as glucocorticoids to prevent acute exacerbation of COVID-19 may also worsen metabolic profiles, such as blood glucose, in patients with COVID-19 [3,4]. Moreover, recent evidence suggests that COVID-19 may affect various organs in the body even after 30 days, the acute phase of infection, increasing the risk of non-communicable diseases in the extrapulmonary organs [5]. Several studies have shown that autoimmunity against pancreatic beta cells increased after COVID-19 which may also increase the risk of type 1 diabetes [6,7]. Additionally, small-scale studies have suggested that COVID-19 might increase the incidence of type 2 diabetes mellitus (T2DM) [8,9], and recent large cohort studies from national healthcare databases have also shown a risk of developing diabetes [10-12]. Despite this growing evidence, there are few large-scale nationwide studies on the effects of COVID-19 on the incidence of T2DM across all ages and sexes. Hence, a detailed assessment of the risk and burden of T2DM is required to establish a post-acute healthcare strategy for COVID-19. This correlation can also provide critical evidence for understanding the pathophysiology of various organs after an acute phase of COVID-19. Accordingly, this study aimed to investigate the association between COVID-19 and the incidence of newly diagnosed T2DM, using a representative nationwide population-based healthcare database in Korea.
This retrospective cohort study was performed using the Health Insurance Review and Assessment Service (HIRA) database of Korea. Briefly, all hospitals and clinics in Korea provide the medical records of all patients covered by the National Health Insurance and Medical Aid to the HIRA office for reimbursement. Therefore, the HIRA claims database includes more than 99% of the Korean population and is composed of six domains (general information, healthcare services, diagnosis, outpatient prescriptions, drug information, and provider information). COVID-19-related outcomes and death records of patients with COVID-19 from January 20th (the date of the first confirmed case) were also shared with the researchers in South Korea. All patient-related records used in this study were anonymized to ensure confidentiality. This study was approved by the Institutional Review Board of the HIRA (IRB No. 2022-082). The requirement for informed consent was waived because all data were anonymized.
All patients with COVID-19 who had the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) diagnostic codes indicating COVID-19 of B34.2 (coronavirus infection, unspecified site), U18.1 (novel coronavirus infection), U07.1 (COVID-19, virus identified), U07.2 (COVID-19, virus not identified) between January 2020 and September 2021 were included in this study. Generally, the acute infectious phase of COVID-19 is considered to occur within 30 days of diagnosis. Thus, 30 days after the diagnosis of COVID-19 was used as the index date. A total of 432,795 adults aged 19 to 89 years were diagnosed with COVID-19 during the study period. Among them, those who were diagnosed with diabetes (ICD codes: E10–E14) and visited the clinic more than twice a year before the index date or died in the acute period were excluded (n=84,615). Finally, 348,180 individuals were included in the COVID-19 exposure group (COVID-19+); the COVID-19 non-exposure group (COVID-19−), which is the control group, included age- and sex-matched subjects with those of COVID-19+ at the index date in a 1:3 ratio with the general population without a history of both COVID-19 and any type of diabetes (n=1,044,540) (Fig. 1). Newly diagnosed T2DM was defined as patients who were first diagnosed with T2DM after the index date and visited a clinic more than twice a year for T2DM. The ICD-10 codes for T2DM included E11–E14. Each individual was followed from the index date to the earliest occurrence of newly diagnosed T2DM, death, or the end of the study period (April 30, 2022).
The primary outcome of this study was the incidence ratio of newly diagnosed T2DM in the COVID-19+ group compared to that in the COVID-19− after the index date. As a secondary outcome, we compared the incidence of newly diagnosed T2DM according to the severity of COVID-19. For this analysis, the patients in the COVID-19+ group were categorized into non-hospitalized, hospitalized, or admitted to an intensive care unit (ICU) for the treatment of COVID-19 during the acute phase. We also compared the incidence of newly diagnosed T2DM with or without glucocorticoid use, categorized according to the dosage, during the acute phase. A history of hypertension and dyslipidemia was investigated to determine the most common comorbidities of T2DM. Hypertension (ICD-10 codes: I10–I15) and dyslipidemia (ICD-10 codes: E78) were defined as the presence of appropriate ICD-10 codes at least twice on a different date within 1 year prior to the index date.
The characteristics of patients in both groups were presented as actual numbers with percentage (%) for categorical variables and mean with standard deviation for continuous variables. Independent t tests and chi-square analyses were used to compare the clinical characteristics of the two groups. A Cox proportional hazards regression model for matched data after adjustment for comorbidities was used to evaluate the relative hazard for events in the COVID-19+ group considering the control group as a reference, and the relative hazards are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The cumulative incidence of newly diagnosed T2DM according to the occurrence of COVID-19 was analyzed using Kaplan-Meier estimates, and the log-rank test was performed to compare differences among the groups. All statistical analyses were performed using R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria), and a two-sided P<0.05 was considered statistically significant.
The demographic characteristics, comorbidities, and follow-up period of COVID-19+ and COVID-19− are provided in Table 1. A total of 1,392,720 Korean adults were recruited from January 1, 2020, to September 30, 2021; 348,180 were COVID-19+ and 1,044,540 were COVID-19−. The mean age at infection was 43.3 years, and the proportion of males was 49.7%. The prevalence of hypertension in the COVID-19+ and COVID-19− groups was 15.3% and 12.3%, respectively, and that of dyslipidemia was 18.7% and 14.0%, respectively. Both diseases were higher in COVID-19+ patients despite the age and sex ratios of the two groups being the same. Among the patients in the COVID-19+ group, 84.1% never used glucocorticoids, 5.1% were given prednisolone at an equivalent dose of <60 mg, 7.5% at 60 to 419 mg, and 3.3% at ≥420 mg. The median follow-up duration was 11.1 months (interquartile range [IQR], 7.8 to 15.7) in the COVID-19+ and 11.2 months (IQR, 7.8 to 15.7) in the COVID-19− groups.
In the post-acute period of COVID-19, the incidence of newly diagnosed T2DM in the COVID-19+ group was 2.95 per 100 person-years (95% CI, 2.90 to 3.01), and in the COVID-19− group was 2.07 per 100 person-years (95% CI, 2.05 to 2.10). The patients in the COVID-19+ group had an increased risk (HR, 1.42; 95% CI, 1.39 to 1.46) of newly diagnosed T2DM compared to those in the COVID-19− group. Even when adjusted for hypertension and dyslipidemia, the adjusted HR was 1.30 (95% CI, 1.27 to 1.33), indicating an increased risk of newly diagnosed T2DM in the COVID-19+ group (Table 2). This increased risk of developing T2DM for patients in the COVID-19+ group persisted for about 2 years: HR, 1.25 (95% CI, 1.21 to 1.29) at 6 months; HR, 1.26 (95% CI, 1.21 to 1.31) at 1 year; HR, 1.49 (95% CI, 1.41 to 1.59) at 18 months; HR, 1.75 (95% CI, 1.57 to 1.95) at 2 years (Fig. 2).
Next, we evaluated whether COVID-19 affects the incidence of newly diagnosed T2DM according to sex, age, COVID-19 severity, and glucocorticoid use in the acute phase. The patients in the COVID-19+ group had an increased risk of newly diagnosed T2DM compared to those in the COVID-19− group, for both males (adjusted HR, 1.32; 95% CI, 1.28 to 1.36) and females (adjusted HR, 1.29; 95% CI, 1.25 to 1.33). The patients in the COVID-19+ group also had an increased risk of newly diagnosed T2DM compared to those in the COVID-19− group, irrespective of their age (i.e., in both young adults [<50 years old] and older adults [≥50 years old]). However, the HR of young adults (adjusted HR, 1.54; 95% CI, 1.48 to 1.61) was slightly higher than that of older adults (adjusted HR, 1.19; 95% CI, 1.16 to 1.22). The HRs increased with the increasing severity of COVID-19 in the acute phase: non-hospitalized (adjusted HR, 1.14; 95% CI, 1.08 to 1.19), hospitalized (adjusted HR, 1.34; 95% CI, 1.30 to 1.38), and ICU-admitted patients (adjusted HR, 1.78; 95% CI, 1.59 to 1.99). However, there was an increased risk of newly diagnosed T2DM in all subgroups classified according to the severity of COVID-19 in the acute phase, even in mild cases that did not require hospitalization. In addition, the HRs were slightly higher for the glucocorticoid users than non-users in the COVID-19+ group, and this increase was not dose-dependent. However, the HR of the glucocorticoid non-users was also higher (adjusted HR, 1.29; 95% CI, 1.25 to 1.32) (Table 3).
To the best of our knowledge, our analysis is the first to examine the impact of COVID-19 on the incidence of T2DM using a representative nationwide healthcare database that includes most Koreans. We analyzed the HRs of T2DM incidence in patients with COVID-19 using a control group matched for age, sex, and the presence of metabolic comorbidities such as hypertension and dyslipidemia.
Our findings show that the risk and burden of incident T2DM increased in the post-acute phase of COVID-19 among Korean adults, which is consistent with a recent analysis using the national healthcare database from the U.S. Veterans Health Administration and another by the U.S. Centers for Disease Control (US-CDC) [11,12]. In recent cohort studies, patients with COVID-19 also had a higher risk of developing T2DM than those with other acute upper respiratory infections [10,13]. In contrast, a recent cohort study conducted in the UK showed that the incidence of diabetes increased up to 12 weeks, but not from 13 to 52 weeks, after COVID-19 [14]. However, the increased risk of developing T2DM in patients with COVID-19 persisted from 13 weeks to approximately 2 years in our study. Therefore, T2DM needs to be considered as a component of the consequences of long COVID-19 and attention should be paid in terms of diabetic management.
In subgroup analysis, there was a significant increase in the incidence of T2DM according to the severity of COVID-19 in the acute phase, which was also consistent with other cohort studies [11,12]. The higher incidence of T2DM in patients who were hospitalized might be related to changes in the counterregulatory hormones of insulin, such as catecholamine or cortisol during the acute infection, or drugs such as glucocorticoids used to prevent acute exacerbation of COVID-19 [15]. However, there was a significantly increased risk of T2DM even in asymptomatic or mild COVID-19 patients who did not require hospitalization. The burden is substantial and not trivial, although it shows a lower HR, given the high prevalence of COVID-19 and that most of these patients are not hospitalized. Taken together, these results suggest that long-term follow-up for T2DM might be necessary, even in patients who have experienced mild COVID-19 in the acute phase.
Currently, standard care for patients requiring hospitalization and oxygenation for severe COVID-19 includes glucocorticoid therapy [16]. The use of glucocorticoids might increase the risk of hyperglycemia and new-onset diabetes, depending on the dose and duration [17]. In the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial, which included 24% of patients with diabetes, hyperglycemia due to short-term glucocorticoid use was observed only in two among 2,100 patients [4]. However, there are no long-term and prospective studies on the effects of glucocorticoid use on the incidence of new-onset diabetes during the acute phase of COVID-19. In our study, glucocorticoid users had a higher HR than non-users, but the increase was not dose-dependent. Noteworthily, glucocorticoid non-users also showed an increased HR (adjusted HR, 1.29; 95% CI, 1.25 to 1.32). This might be because the incidence and severity of COVID-19 contribute to the increased risk of developing T2DM, independently of glucocorticoid use.
The adjusted HR for the increased risk of developing T2DM due to COVID-19 was 1.54 for young adults (≤50 years old) and 1.19 for older people (>50 years old), which was higher in younger adults, unlike in other studies [11,12]. Contrastingly, in the US-CDC report, the adjusted HR was 1.39 (95% CI, 1.35 to 1.44) for young adults (<65 years old) and 1.53 (95% CI, 1.48 to 1.59) for the elderly (≥65 years old), which was higher in the elderly [12]. These findings can be considered primarily attributable to the much lower incidence of T2DM in young adults aged ≤50 years in our cohort. In the US-CDC report, the incidence of T2DM per 100 person-years was 2.28 in the COVID-19− group and 3.24 in the COVID-19+ roup among young adults (<65 years) [12]. Although not shown, our study showed that the incidence of T2DM per 100 person-years in younger adults (≤50 years old) was 0.89 (95% CI, 0.87 to 0.91) in the COVID-19− group and 1.56 (95% CI, 1.51 to 1.61) in the COVID-19+ group, respectively. As a result, the excess burden for the incidence of T2DM due to COVID-19 is 0.67 per 100 personyears, which is lesser than the 0.96 in the US-CDC report. Additionally, it is lesser than that of the older patients in our cohort, which is 1.31 per 100 person-years: 4.32 (95% CI, 4.26 to 4.39) in the COVID-19− group and 5.63 (95% CI, 5.50 to 5.77) in the COVID-19+ group, although this is not indicated in our study results.
Our study has a few limitations. We defined T2DM only using the ICD-10 code for diagnosis, although the same was applied to both groups. Additionally, our observations were too brief to evaluate the risk given the insidious onset of T2DM. Thus, our analysis might have underestimated the risk of T2DM development, and long-term studies with more precisely defined T2DM are warranted. Next, we generated a control group matched only for age and sex. The risk factors for T2DM could be significantly different between the two groups. Several studies have shown that the morbidity and severity of COVID-19 are higher in individuals with multiple underlying diseases [18-20]. Prediabetes is considered a risk state that defines a high chance of developing diabetes [21]. Thus, we also considered investigating the prevalence of prediabetes in both groups. However, R730 of the ICD-10 code for defining patients with prediabetes in the HIRA database is not well used for research due to its low reliability. Instead, we investigated and adjusted for the presence of hypertension and dyslipidemia, which are representative comorbidities of T2DM. Our study included approximately half of those under the age of 40, and both T2DM and severe COVID-19 incidence in young people are known to increase linearly with body weight [22,23]. However, we were unable to retrieve the weight of the subjects from the database. Therefore, it is possible that our study did not sufficiently control for other risk factors, although the results were adjusted for hypertension and dyslipidemia. Finally, the biggest limitation of most observational studies, including ours, is that subsequent medical status is more likely to be assessed and documented in people with COVID-19 than in those without a history of COVID-19. Similarly, the higher the severity of COVID-19, the higher the HR. Indeed, the incidence of most conditions was higher in patients with COVID-19 in the US-CDC report [12]. Therefore, large-scale prospective cohort studies with carefully curated controls and long-term assessments of health status after COVID-19 are needed.
In conclusion, the risk and burden of T2DM increased in the post-acute period of COVID-19, which is dependent on the severity of COVID-19 in the acute phase. Therefore, a strategy for the screening and appropriate management of T2DM is necessary for overall healthcare management after the acute phase of COVID-19.
ACKNOWLEDGMENTS
This study was supported by the Big Data Research Funds from the Korean Endocrine Society.
REFERENCES
1. Steenblock C, Schwarz PE, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021; 9:786–98.

2. Garces CP, Oliveira E Silva L, Nunes SM, Cheik NC. Effects of social distancing caused by the COVID-19 pandemic on physical activity level, sitting time, and binge eating: a comparison between overweight/obese and normal-weight adults. Sport Sci Health. 2022; 18:1505–12.

3. Shao S, Yang Q, Pan R, Yu X, Chen Y. Interaction of severe acute respiratory syndrome coronavirus 2 and diabetes. Front Endocrinol (Lausanne). 2021; 12:731974.

4. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384:693–704.

5. Louis TJ, Qasem A, Abdelli LS, Naser SA. Extra-pulmonary complications in SARS-CoV-2 infection: a comprehensive multi organ-system review. Microorganisms. 2022; 10:153.

6. Cinek O, Slavenko M, Pomahacova R, Venhacova P, Petruzelkova L, Skvor J, et al. Type 1 diabetes incidence increased during the COVID-19 pandemic years 2020-2021 in Czechia: results from a large population-based pediatric register. Pediatr Diabetes. 2022; 23:956–60.
7. Mastromauro C, Blasetti A, Primavera M, Ceglie L, Mohn A, Chiarelli F, et al. Peculiar characteristics of new-onset type 1 diabetes during COVID-19 pandemic. Ital J Pediatr. 2022; 48:26.
8. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021; 23:870–4.
9. Montefusco L, Ben Nasr M, D’Addio F, Loretelli C, Rossi A, Pastore I, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021; 3:774–85.

10. Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years: United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022; 71:59–65.

11. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022; 10:311–21.

12. Bull-Otterson L, Baca S, Saydah S, Boehmer TK, Adjei S, Gray S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years: United States, March 2020-November 2021. MMWR Morb Mortal Wkly Rep. 2022; 71:713–7.
13. Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after COVID-19. Diabetologia. 2022; 65:949–54.

14. Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection: a matched cohort study in the UK. PLoS Med. 2022; 19:e1004052.

15. Knebusch Toriello N, Prato Alterio NM, Ramirez Villeda LM. Newly diagnosed diabetes mellitus during COVID-19: the new pandemic: a literature review. Curr Trop Med Rep. 2022; 9:250–6.

16. Johns M, George S, Taburyanskaya M, Poon YK. A review of the evidence for corticosteroids in COVID-19. J Pharm Pract. 2022; 35:626–37.

17. Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab (Seoul). 2017; 32:180–9.

18. Ji W, Lee R, Huh K, Kang M, Hwang IC, Radnaabaatar M, et al. Overweight and obesity are risk factors for coronavirus disease 2019: a propensity score-matched case-control study. Endocrinol Metab (Seoul). 2021; 36:196–200.

19. You JH, Lee SA, Chun SY, Song SO, Lee BW, Kim DJ, et al. Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol Metab (Seoul). 2020; 35:901–8.

20. Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020; 35:e237.

21. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012; 379:2279–90.

Fig. 1.
Study flow for cohort selection and matched control. COVID-19, coronavirus disease 2019; COVID-19+, COVID-19 exposure group; COVID-19−, COVID-19 non-exposure control group; DM, diabetes mellitus; T2DM, type 2 diabetes mellitus.
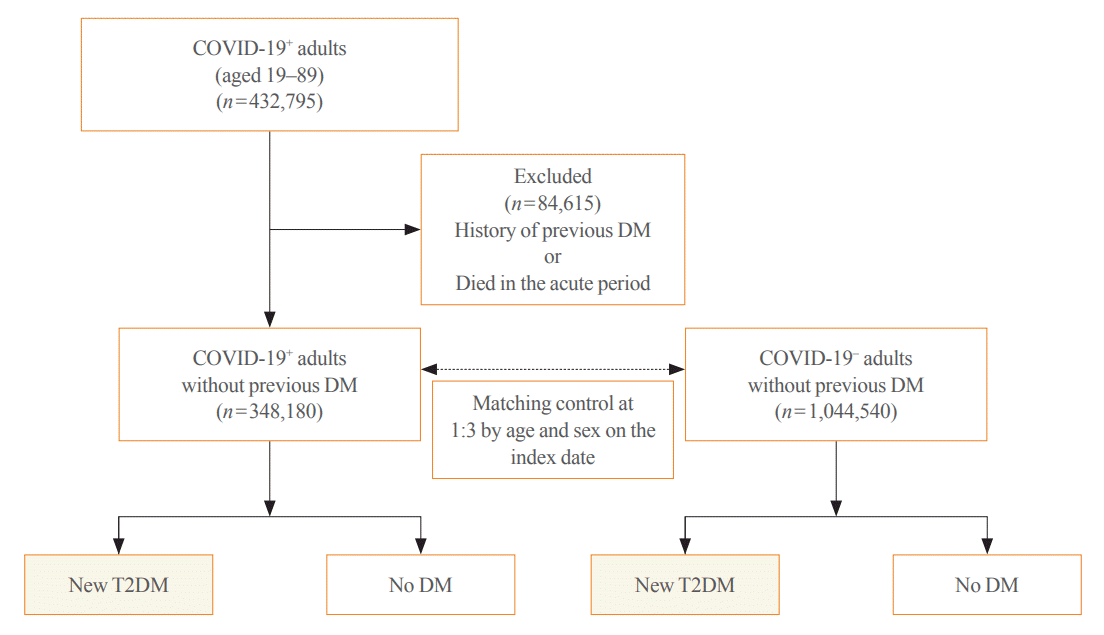
Fig. 2.
Kaplan-Meier curve for the cumulative incidence of newly diagnosed type 2 diabetes mellitus according to the status of coronavirus disease 2019 (COVID-19). COVID-19+, COVID-19 exposure group; COVID-19−, COVID-19 non-exposure control group.
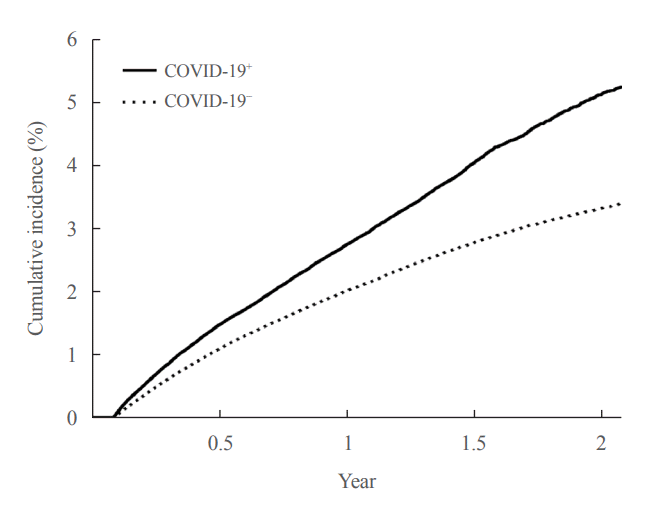
Table 1.
Baseline Characteristics of COVID-19+ and Age- and Sex-Matched COVID-19− Group
| Characteristic | Total | COVID-19+ | COVID-19− | P value | |
|---|---|---|---|---|---|
| No. of subjects | 1,392,720 | 348,180 | 1,044,540 | ||
| Age, yr | 43.3±16.3 | 43.3±16.3 | 43.3±16.3 | >0.999 | |
| 19–29 | 25.5 | 25.5 | 25.5 | ||
| 30–39 | 20.4 | 20.4 | 20.4 | ||
| 40–49 | 19.5 | 19.5 | 19.5 | ||
| 50–59 | 17.1 | 17.1 | 17.1 | ||
| 60–69 | 10.6 | 10.6 | 10.6 | ||
| 70–89 | 7.0 | 7.0 | 7.0 | ||
| Male sex, % | 49.7 | 49.7 | 49.7 | >0.999 | |
| Comorbidities, % | |||||
| Hypertension | 13.1 | 15.3 | 12.3 | <0.001 | |
| Dyslipidemia | 15.2 | 18.7 | 14.0 | <0.001 | |
| Glucocorticoid usea, % | |||||
| None | 84.1 | ||||
| <60 mg | 5.1 | ||||
| 60–419 mg | 7.5 | ||||
| ≥420 mg | 3.3 | ||||
| Follow-up, mo | |||||
| Mean±SD | 12.5±5.8 | 12.4±5.8 | 12.6±5.8 | <0.001 | |
| Median (IQR) | 11.2 (7.8–15.7) | 11.1 (7.8–15.7) | 11.2 (7.8–15.7) | ||
Table 2.
Risk and Burden of Newly Diagnosed T2DM in the COVID-19 Group Compared with the Control Group
| Variable | Total | COVID-19+ | COVID-19− | P value |
|---|---|---|---|---|
| Total no. of subjects | 1,392,720 | 348,180 | 1,044,540 | |
| Newly diagnosed T2DM | 33,339 | 10,668 | 22,671 | |
| Follow-up duration, person-yr | 1,455,356 | 361,223 | 1,094,133 | |
| Incidence rate, /100 person-yr | 2.29 (2.27–2.32)b | 2.95 (2.90–3.01)b | 2.07 (2.05–2.10)b | |
| HR | 1.42 (1.39–1.46)b | 1 (reference) | <0.001 | |
| Adjusted HRa | 1.30 (1.27–1.33)b | <0.001 |
Table 3.
Risks of Newly Diagnosed Type 2 Diabetes according to the Severity of COVID-19 in the Acute Phase, Sex, and Age Compared with the Control Group
| Variable | HR (95% CI) | P value | Adjusted HR (95% CI)a | P value | |
|---|---|---|---|---|---|
| Severity of COVID-19b | |||||
| Non-hospitalized | 1.25 (1.19–1.31) | <0.001 | 1.14 (1.08–1.19) | <0.001 | |
| Hospitalized, but not ICU | 1.47 (1.43–1.50) | <0.001 | 1.34 (1.30–1.38) | <0.001 | |
| ICU-admitted | 1.98 (1.77–2.22) | <0.001 | 1.78 (1.59–1.99) | <0.001 | |
| Sexb | |||||
| Male | 1.46 (1.41–1.51) | <0.001 | 1.32 (1.28–1.36) | <0.001 | |
| Female | 1.39 (1.34–1.43) | <0.001 | 1.29 (1.25–1.33) | <0.001 | |
| Age, %c | |||||
| <50 yr | 1.75 (1.69–1.83) | <0.001 | 1.54 (1.48–1.61) | <0.001 | |
| ≥50 yr | 1.30 (1.26–1.34) | <0.001 | 1.19 (1.16–1.22) | <0.001 | |
| Glucocorticoid used, % | |||||
| None | 1.39 (1.36–1.43) | <0.001 | 1.29 (1.25–1.32) | <0.001 | |
| <60 mg | 1.66 (1.51–1.82) | <0.001 | 1.43 (1.30–1.57) | <0.001 | |
| 60–419 mg | 1.52 (1.41–1.63) | <0.001 | 1.37 (1.27–1.47) | <0.001 | |
| ≥420 mg | 1.50 (1.36–1.65) | <0.001 | 1.34 (1.21–1.48) | <0.001 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download