Abstract
Plasmacytoid dendritic cells (pDCs) are type I interferon-producing cells that modulate immune responses. There are two types of pDC neoplasms: 1) mature pDC proliferation (MPDCP) associated with myeloid neoplasm and 2) blastic pDC neoplasm (BPDCN). MPDCP is a clonal expansion of mature pDCs that is predominantly associated with chronic myelomonocytic leukemia. In contrast, BPDCN is a clinically aggressive myeloid malignancy involving the skin, bone marrow, lymphatic organs, and central nervous system. There are various types of skin lesions, ranging from solitary brown or violaceous to disseminated cutaneous lesions, which often spread throughout the body. The expression of CD4, CD56, CD123, and pDC markers (TCL-1, TCF4, CD303, and CD304, etc.) are typical immunophenotype of BPDCN. Historically, BPDCN treatment has been based on acute leukemia regimens and allogeneic hematopoietic cell transplantation in selected patients. Recent advances in molecular biology and genetics have led to the development of targeted agents, such as tagraxofusp (a recombinant fusion protein targeting CD123), anti-CD123 CAR-T cells, XmAb14045, and IMGN632. Lastly, this review provides a comprehensive overview of pDC neoplasms.
In 1958, plasmacytoid dendritic cells (pDCs) were first described as unique dendritic cells that modulate innate and adaptive immunity [1, 2]. Dendritic cells are divided into myeloid dendritic cells and pDCs, which exhibit cytomorphologic plasmacytoid features and produce interferon-α [3]. pDCs may accumulate abnormally in the reactive lymph nodes and skin of patients with Kikuchi lymphadenitis, Castleman hyaline vascular disease, lupus erythematosus, and myeloid neoplasms [3]. The uncertain origins, features, and functions of pDCs have been studied for several decades. Recently, pDC neoplasms were placed in the category of myeloid and histiocytic/dendritic neoplasms in the 5th edition of the World Health Organization classification because of the clonal proliferation of the precursors of pDCs derived from common myeloid progenitors [4]. There are two types of pDC neoplasms originating from pDCs: 1) mature pDC proliferation (MPDCP) associated with myeloid neoplasms and 2) blastic pDC neoplasm (BPDCN). This review provides a comprehensive overview of pDC neoplasms with a particular focus on BPDCN.
MPDCP associated with myeloid neoplasm manifests as nodules or aggregates of mature pDCs in the lymph nodes, skin, and bone marrow [4, 5]. MPDCP represents a morphologically mature, low-grade proliferation of pDCs, characterized by the expression of pDC markers (including CD123, CD303, CD304, TCF4), a low proliferation index (<10% Ki-67), and the absence or low expression of CD56 [6]. MPDCP is predominantly associated with chronic myelomonocytic leukemia (CMML) [7]. MPDCP shares clonality with the accompanying myeloid neoplasm and presents with similar clinical characteristics. Treatment is generally determined based on the underlying myeloid neoplasm.
However, pDCs have been associated with poor outcomes in patients with myeloid neoplasms. There is a correlation between activating RAS mutations, regulatory T cell accumulation, and a higher risk of acute leukemia transformation [7]. Additionally, acute myeloid leukemia (AML) can have clonally expanded pDCs (pDC-AML) that exhibit frequent mutations in RUNX1 [8]. MPDCP associated with myeloid neoplasms may be better defined when expressed as AML (pDC-AML), myelodysplastic syndrome (pDC-MDS) or CMML (pDC-CMML) [7-10].
BPDCN is a rare disease characterized by heterogeneous clinical features, complex diagnostic processes, and numerous changes in nomenclature and classification over the decades. Therefore, there is a lack of knowledge regarding BPDCN. In the United States, BPDCN account for only 0.44% of all hematological malignancies, 0.7% of all cutaneous leukemias/lymphomas, and 0.04 cases per 100,000 [11]. There is an extreme male predominance of 2–4:1 and a median age of 53 years [12]; a bimodal pattern was observed in older (>60 yr) and younger (<20 yr) individuals [12]. Additionally, the male predominance has been associated with ZRSR2 mutations on the X chromosome [13].
A BPDCN is a clinically aggressive myeloid malignancy that involves the skin, bone marrow, lymphatic organs, and central nervous system (CNS)/cerebrospinal fluid (CSF) [14, 15]. Most patients (80%) have skin involvement and 50% have only skin lesions. Skin lesions range from solitary brown or violaceous bruise-like lesions, erythematous patches, plaques, and ulcerative lesions to disseminated cutaneous spread [16, 17]. Most patients present with localized skin lesions that progressively spread throughout the body, whereas some patients initially present with leukemia without any skin lesions. More than 60% of patients have bone marrow involvement and diffuse involvement, and progressive cytopenia is common [16, 18]. More than 20% of patients have a prior or concomitant myeloid malignancy, such as AML, MDS, or CMML [15, 16]. BPDCN rapidly disseminate in the bone marrow, lymph nodes, liver, spleen, and CNS. In one study, flow cytometric analysis identified CSF positivity in 60% of patients with BPDCN at diagnosis and in 100% of patients at relapse [19]. Thus, the clinical manifestations of BPDCN are highly variable and often misdiagnosed or delayed. A BPDCN has a poor prognosis, with a median overall survival (OS) of 2 years [16].
BPDCN has no known environmental or hereditary genetic factors that contribute to its development, although it frequently exhibits abnormal karyotypes on 5q, 12p, 17p, 13q, 6q, 15q, and 9 monosomy [20]. The 9p21.3 deletion is associated with a poor prognosis [21]. Furthermore, MYC, MYB, and MLL rearrangement is common in BPDCN [22-24]. In recent studies, BPDCN exhibited recurrent mutations in ASXL1, IDH1-2, IKZF1-3, NPM1, TET1-2, TP53, U2AF1, and ZEB2, as well as the overexpression of IncRNA-3q or TCF4 [25-27]. Genetic abnormalities are heterogeneous; therefore, their molecular mechanisms and prognostic significance remain controversial.
Its diagnostic workup is summarized in Table 1. The diagnosis of BPDCN is usually initiated with suspicion regarding patients with skin lesions or progressive pancytopenia. Although the shape, size, color, and pattern of BPDCN skin lesions vary, their morphological features and immunohistochemical expression patterns are typical. Immature blastic cells infiltrate deeply into the dermis, sparing the epidermis and preventing angioinvasion, coagulative necrosis, or inflammatory cells [28]. These cells typically express CD4 and CD56, as well as at least one of the pDC-associated markers (CD123, CD303, CD304, TCL1, and TCF4) in the absence of lineage-specific markers for myeloids, T lymphocytes, B lymphocytes, or NK cells [28, 29]. Additionally, there is a pearl-necklace appearance of blast cells in bone marrow puncture smears with tiny vacuoles extending in a pseudopod-like pattern along the cytoplasmic membrane [29]. They are medium-sized cells with scant cytoplasm, fine chromatin, and irregular nucleoli [29]. However, there are a few cases in which is negative for CD4, CD56, or both.
There is a need for a multidisciplinary approach to the diagnosis of BPDCN. As shown in Table 3, BPDCN can be distinguished from other CD56-positive hematologic malignancies that affect the skin, such as leukemia cutis, myeloid sarcoma, extranodal NK/T cell lymphoma, and cutaneous T cell lymphoma [29].
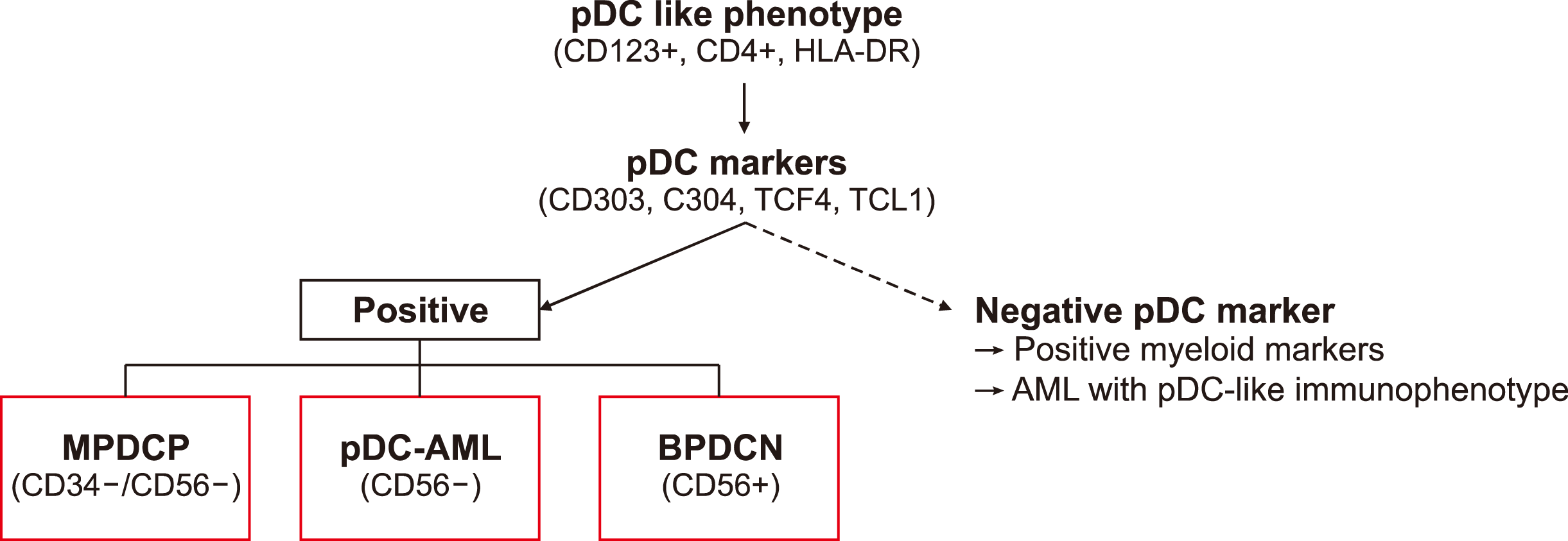
BPDCN can occur as an isolated disease or in the context of other hematologic neoplasms. However, the relationship between BPDCN and other myeloid malignancies has not yet been elucidated. Therefore, the differential diagnosis of BPDCN is necessary when pDCs are present in patients with myeloid malignancies or ambiguous immunophenotypes. This is based on CD56 positivity and the presence of pDC markers (Fig. 1) [5]. Compared to BPDCN, pDC-AML exhibits RUNX1 mutations, fewer skin lesions, and expresses CD34-positive and CD56-negative pDCs [8]. Furthermore, NPM1-mutated AML with a pDC-like phenotype expresses CD123-positive and other pDC marker-negative cells and has poor outcomes [30].
There is currently no standard treatment for BPDCN. Historically, BPDCN treatments have been based on multi-agent chemotherapy regimens for lymphoma, acute lymphoblastic leukemia, and AML [28]. In addition, acute leukemia regimens achieve high complete response (CR) rates ranging from 40–90% and allogeneic hematopoietic cell transplantation (allo-HCT) can result in durable remission in some patients. However, their rarity and heterogeneity make it difficult to determine the most effective therapeutic strategies [11]. Owing to recent advances in molecular biology and genetics, targeted treatment strategies have been developed. In December 2018, the FDA approved tagraxofusp, a first-in-class CD123-targeting therapy for treatment-naïve or relapsed/refractory BPDCN [31]. However, unfit, relapsed, or refractory patients continue to require effective therapeutic strategies.
The most common induction regimens for BPDCN are CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine); high doses of methotrexate combined with asparaginase; and the 7+3 regimen (cytarabine combined with idarubicin or daunorubicin) [32]. Several retrospective studies have revealed better outcomes with lymphoid-type regimens than with myeloid regimens; however, only allo-HCT was associated with prolonged OS [15, 16]. Recently, the French BPDCN network and the German Society of Hematology and Oncology recommended acute leukemia regimens rather than CHOP or CHOP-like regimens [14, 32]. A prospective phase II trial to evaluate the effectiveness of three cycles of methotrexate, L-asparaginase, idarubicin, and dexamethasone, followed by allo-HCT, for eligible patients is ongoing (NCT03599960).
Allo-HCT is the standard consolidation treatment for fit patients with BPDCN who achieve CR1 [33, 34]. Based on a meta-analysis of 128 patients who underwent allo-HCT, the pooled OS was 70% in CR1 and 7% beyond CR1 [35]. However, no randomized controlled trials have compared allo-HCT with other therapies, especially in the era of novel therapies. A North American collaborative study revealed similar outcomes between myeloablastive conditioning (MAC) and reduced-intensity conditioning regimens [36]. Data from the EBMT registry indicate MAC based on TBI results in long-term remission after allo-HCT for BPDCN [33]. Due to limited data, autologous HCT may lack efficacy against BPDCN [36]. However, patients who are ineligible for allo-HCT may benefit from autologous HCT [33, 37].
Tagraxofusp, an interleukin (IL) 3-diphtheria toxin fusion protein, targets the IL3 receptor alpha subunit or CD123, which is highly expressed on BPDCN cells [38]. In patients with treatment-naïve BPDCN, tagraxofusp resulted in a 75% overall response (CR: 57%) with a median OS of 15.8 months (range, 9.7 to 25.8) [39]. In the previously treated group, 67% of the patients achieved an overall response with a median OS of 8.5 months [31, 39]. The median response durations for the treatment-naïve and previously treated BPDCN groups were 24.9 months (range, 3.8 to not reached) and 2.8 months (range, 0.7 to 14.0), respectively [31]. The most common adverse events were the elevation of liver enzymes, hypoalbuminemia, thrombocytopenia, peripheral edema, and capillary leak syndrome (CLS) [31]. CLS was the most serious, resulting in three deaths [31]. CLS is treated by holding tagraxofusp and administering intravenous albumin or steroids and volume control [39]. In addition to tagraxofusp (a recombinant fusion protein targeting CD123), CD28/4-1BB CD123 CAR T cells, UCART123 (anti-CD123 CAR-T cells), XmAb14045 (a CD123/CD3 bispecific antibody), and IMGN632 (CD123 antibody-drug conjugates) have been investigated [24, 40-42]. IMGN632 is a humanized monoclonal antibody that targets CD123 fused to a potent DNA-alkylating agent. A phase I clinical trial of IMGN632 in CD123-positive hematologic malignancies is currently ongoing (NCT03386513).
BPDCN highly express anti-apoptotic protein B cell leukemia/lymphoma-2 (BCL-2) [43]. Several cases have revealed the efficacy of venetoclax, a BCL-2 inhibitor [43, 44]. In addition, epigenetic alterations are common in BPDCN, and the efficacy of 5-azacytidine (5-Aza) in patients with BPDCN has been reported [45]. However, monotherapy does not demonstrate long-term efficacy. Venetoclax in combination with hypomethylating agents or tagraxofusp for BPDCN has been reported to be synergistic [46, 47]. Therefore, several clinical trials have targeted these mechanisms, including those using 5-Aza, venetoclax, and tagraxofusp.
Several case reports have suggested a potential role of myeloma-based therapy in the treatment of BPDCN. Bortezomib for the constitutive activation of the NF-κB pathway, lenalidomide for IKZF1 depletion, and daratumumab, which targets CD38 overexpression in BPDCN, were used [48-50]. Marmouset et al. [49] described a promising combination of bortezomib/lenalidomide and dexamethasone in 2019; Mirgh et al. [51] demonstrated the efficacy of daratumumab in combination with bortezomib in CD38-positive BPDCN.
The genetic landscape of BPDCN is characterized by recurrent MYB/MYC rearrangements, epigenetic alterations, the downregulation of the liver X receptor (LXR), and the dysregulation of the TCF4-/BRD4-dependent transcriptional network [24, 52]. NF-κB inhibitors, BRD4 inhibitors, and LXR agonists are potential therapeutic targets for BPDCN. Furthermore, the use of bromodomain and extra-terminal motif inhibitors (BETi), which antagonize the TCF4 amplification loop, can induce blastic pDC apoptosis [26]. Further research is required to determine the most effective combination or sequential therapies for BPDCN, including allo-HCT and novel agents.
Although our understanding of pDC neoplasm is improving, several issues remain unclear. A deeper understanding of heterogeneous genetic profiles is required. Tagraxofusp, a drug targeting CD123, has been approved by the FDA for BPDCN, and other drugs targeting CD123 are under investigation. Additionally, venetoclax and other hypomethylating agents are clinically beneficial. Tagraxofusp or chemotherapy followed by allo-HCT in CR1 is the best treatment strategy for fit patients with BPDCN, and targeted therapy may be effective in unfit or relapsed patients. However, further studies are needed to identify other potential therapeutic strategies.
REFERENCES
1. Lennert K, Remmele W. 1958; Karyometrische untersuchungen an lymphknotenzellen des menschen. Acta Haematol. 19:99–113. DOI: 10.1159/000205419. PMID: 13520253.
2. Chaperot L, Bendriss N, Manches O, et al. 2001; Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 97:3210–7. DOI: 10.1182/blood.V97.10.3210. PMID: 11342451.
3. Jegalian AG, Facchetti F, Jaffe ES. 2009; Plasmacytoid dendritic cells physiologic roles and pathologic states. Adv Anat Pathol. 16:392–404. DOI: 10.1097/PAP.0b013e3181bb6bc2. PMID: 19851130. PMCID: PMC6329307.
4. Khoury JD, Solary E, Abla O, et al. 2022; The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 36:1703–19. DOI: 10.1038/s41375-022-01613-1. PMID: 35732831. PMCID: PMC9252913.
5. El Hussein S, Wang W. 2023; Plasmacytoid dendritic cells in the setting of myeloid neoplasms: diagnostic guide to challenging pathologic presentations. Br J Haematol. 200:545–55. DOI: 10.1111/bjh.18632. PMID: 36606610.
6. Facchetti F, Cigognetti M, Fisogni S, Rossi G, Lonardi S, Vermi W. 2016; Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol. 29:98–111. DOI: 10.1038/modpathol.2015.145. PMID: 26743477.
7. Lucas N, Duchmann M, Rameau P, et al. 2019; Biology and prognostic impact of clonal plasmacytoid dendritic cells in chronic myelomonocytic leukemia. Leukemia. 33:2466–80. DOI: 10.1038/s41375-019-0447-3. PMID: 30894665.
8. Xiao W, Chan A, Waarts MR, et al. 2021; Plasmacytoid dendritic cell expansion defines a distinct subset of RUNX1-mutated acute myeloid leukemia. Blood. 137:1377–91. DOI: 10.1182/blood.2020007897. PMID: 32871587. PMCID: PMC7955409.
9. Roussel X, Garnache Ottou F, Renosi F. 2022; Plasmacytoid dendritic cells, a novel target in myeloid neoplasms. Cancers (Basel). 14:3545–65. DOI: 10.3390/cancers14143545. PMID: 35884612. PMCID: PMC9317563. PMID: 9745cfb676df4414aac46c62e8bf2f26.
10. Zalmaï L, Viailly P, Biichle S, et al. 2021; Plasmacytoid dendritic cells proliferation associated with acute myeloid leukemia: phenotype profile and mutation landscape Loria. Haematologica. 106:2056–3066. DOI: 10.3324/haematol.2020.253740. PMID: 33054115. PMCID: PMC8634182.
11. Pemmaraju N, Kantarjian H, Sweet K, et al. 2022; North American Blastic Plasmacytoid Dendritic Cell Neoplasm Consortium: position on standards of care and areas of need. Blood. 141:567–78. DOI: 10.1182/blood.2022017865. PMID: 36399715.
12. Guru Murthy GS, Pemmaraju N, Atallah E. 2018; Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 73:21–3. DOI: 10.1016/j.leukres.2018.08.014. PMID: 30189324.
13. Togami K, Chung SS, Madan V, et al. 2022; Sex-biased ZRSR2 mutations in myeloid malignancies impair plasmacytoid dendritic cell activation and apoptosis. Cancer Discov. 12:522–41. DOI: 10.1158/2159-8290.CD-20-1513. PMID: 34615655. PMCID: PMC8831459.
14. Garnache-Ottou F, Vidal C, Biichlé S, et al. 2019; How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 3:4238–51. DOI: 10.1182/bloodadvances.2019000647. PMID: 31869411. PMCID: PMC6929390.
15. Laribi K, De Materre AB, Sobh M, et al. 2020; Blastic plasmacytoid dendritic cell neoplasms: results of an international survey on 398 adult patients. Blood Adv. 4:4838–48. DOI: 10.1182/bloodadvances.2020002474. PMID: 33027528. PMCID: PMC7556130.
16. Taylor J, Haddadin M, Upadhyay VA, et al. 2019; Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 134:678–87. DOI: 10.1182/blood.2019001144. PMID: 31243042. PMCID: PMC6706810.
17. Julia F, Petrella T, Beylot-Barry M, et al. 2013; Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 169:579–86. DOI: 10.1111/bjd.12412. PMID: 23646868.
18. Feuillard J, Jacob MC, Valensi F, et al. 2002; Clinical and biologic features of CD4 + CD56 + malignancies. Blood. 99:1556–63. DOI: 10.1182/blood.V99.5.1556. PMID: 11861268.
19. Pemmaraju N, Wilson NR, Khoury JD, et al. 2021; Central nervous system involvement in blastic plasmacytoid dendritic cell neoplasm. Blood. 138:1373–7. DOI: 10.1182/blood.2021011817. PMID: 34098573.
20. Zhang Y, Sokol L. 2022; Clinical insights into the management of blastic plasmacytoid dendritic cell neoplasm. Cancer Manag Res. 14:2107–17. DOI: 10.2147/CMAR.S330398. PMID: 35789956. PMCID: PMC9250318.
21. Lucioni M, Novara F, Fiandrino G, et al. 2011; Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 118:4591–4. DOI: 10.1182/blood-2011-03-337501. PMID: 21900200.
22. Sakamoto K, Katayama R, Asaka R, et al. 2018; Recurrent 8q24 rearrangement in blastic plasmacytoid dendritic cell neoplasm: association with immunoblastoid cytomorphology, MYC expression, and drug response. Leukemia. 32:2590–603. DOI: 10.1038/s41375-018-0154-5. PMID: 29795241.
23. Suzuki K, Suzuki Y, Hama A, et al. 2017; Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 31:1629–33. DOI: 10.1038/leu.2017.101. PMID: 28344318.
24. Wang Y, Xiao L, Yin L, Zhou L, Deng Y, Deng H. 2023; Diagnosis, treatment, and genetic characteristics of blastic plasmacytoid dendritic cell neoplasm: a review. Medicine (Baltimore). 102:e32904. DOI: 10.1097/MD.0000000000032904. PMID: 36800625. PMCID: PMC9936012.
25. Menezes J, Acquadro F, Wiseman M, et al. 2014; Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 28:823–9. DOI: 10.1038/leu.2013.283. PMID: 24072100.
26. Ceribelli M, Hou ZE, Kelly PN, et al. 2016; A druggable TCF4- and BRD4-dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell. 30:764–78. DOI: 10.1016/j.ccell.2016.10.002. PMID: 27846392. PMCID: PMC5175469.
27. Emadali A, Hoghoughi N, Duley S, et al. 2016; Haploinsufficiency for NR3C1, the gene encoding the glucocorticoid receptor, in blastic plasmacytoid dendritic cell neoplasms. Blood. 127:3040–53. DOI: 10.1182/blood-2015-09-671040. PMID: 27060168. PMCID: PMC5043425.
28. Sullivan JM, Rizzieri DA. 2016; Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematology. 2016:16–23. DOI: 10.1182/asheducation-2016.1.16. PMID: 27913457. PMCID: PMC6142460.
29. Venugopal S, Zhou S, El Jamal SM, Lane AA, Mascarenhas J. 2019; Blastic plasmacytoid dendritic cell neoplasm-current insights. Clin Lymphoma Myeloma Leuk. 19:545–54. DOI: 10.1016/j.clml.2019.06.002. PMID: 31281107.
30. Minetto P, Guolo F, Clavio M, et al. 2018; A blastic plasmacytoid dendritic cell neoplasm-like phenotype identifies a subgroup of npm1-mutated acute myeloid leukemia patients with worse prognosis. Am J Hematol. 93:E33–5. DOI: 10.1002/ajh.24956. PMID: 29080220.
31. Pemmaraju N, Lane AA, Sweet KL, et al. 2019; Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 380:1628–37. DOI: 10.1056/NEJMoa1815105. PMID: 31018069.
32. Poussard M, Angelot-Delettre F, Deconinck E. 2022; Conventional therapeutics in BPDCN patients-do they still have a place in the era of targeted therapies? Cancers (Basel). 14:3767–9. DOI: 10.3390/cancers14153767. PMID: 35954431. PMCID: PMC9367503. PMID: 6596e33a50314d07906b7577b6635f61.
33. Bruch PM, Dietrich S, Finel H, et al. 2023; Retrospective analysis of hematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: conditioning intensity matters. Leukemia. 37:465–72. DOI: 10.1038/s41375-022-01782-z. PMID: 36550212.
34. Bashir Q, Milton DR, Popat UR, et al. 2022; Allogeneic hematopoietic cell transplantation for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN). Bone Marrow Transplant. 57:51–6. DOI: 10.1038/s41409-021-01478-5. PMID: 34629467. PMCID: PMC9126091.
35. Kharfan-Dabaja MA, Reljic T, Murthy HS, Ayala E, Kumar A. 2018; Allogeneic hematopoietic cell transplantation is an effective treatment for blastic plasmacytoid dendritic cell neoplasm in first complete remission: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 18:703–9. e1DOI: 10.1016/j.clml.2018.07.295. PMID: 30145196.
36. Kharfan-Dabaja MA, Al Malki MM, Deotare U, et al. 2017; Haematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a North American multicentre collaborative study. Br J Haematol. 179:781–9. DOI: 10.1111/bjh.14954. PMID: 28980314.
37. Aoki T, Suzuki R, Kuwatsuka Y, et al. 2015; Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 125:3559–62. DOI: 10.1182/blood-2015-01-621268. PMID: 25918345.
38. FitzGerald DJ. 2014; Targeted diphtheria toxin to treat BPDCN. Blood. 124:310–2. DOI: 10.1182/blood-2014-06-578633. PMID: 25035145. PMCID: PMC4102704.
39. Pemmaraju N, Sweet KL, Stein AS, et al. 2022; Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 40:3032–6. DOI: 10.1200/JCO.22.00034. PMID: 35820082. PMCID: PMC9462530.
40. Bôle-Richard E, Fredon M, Biichlé S, et al. 2020; CD28/4-1BB CD123 CAR T cells in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 34:3228–41. DOI: 10.1038/s41375-020-0777-1. PMID: 32111969.
41. Cai T, Gouble A, Black KL, et al. 2022; Targeting CD123 in blastic plasmacytoid dendritic cell neoplasm using allogeneic anti- CD123 CAR T cells. Nat Commun. 13:2228. DOI: 10.1038/s41467-022-29669-8. PMID: 35484100. PMCID: PMC9051102. PMID: 28ec66e39af349c1b7a1e856e77e57a0.
42. Angelova E, Audette C, Kovtun Y, et al. 2019; CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica. 104:749–55. DOI: 10.3324/haematol.2018.205252. PMID: 30361418. PMCID: PMC6442980.
43. Montero J, Stephansky J, Cai T, et al. 2017; Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL2 and sensitive to venetoclax. Cancer Discov. 7:156–64. DOI: 10.1158/2159-8290.CD-16-0999. PMID: 27986708. PMCID: PMC5296248.
44. Agha ME, Monaghan SA, Swerdlow SH. 2018; Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 379:1479–81. DOI: 10.1056/NEJMc1808354. PMID: 30304659.
45. Laribi K, Denizon N, Ghnaya H, et al. 2014; Blastic plasmacytoid dendritic cell neoplasm: the first report of two cases treated by 5-azacytidine. Eur J Haematol. 93:81–5. DOI: 10.1111/ejh.12294. PMID: 24571716.
46. Gangat N, Konopleva M, Patnaik MM, et al. 2022; Venetoclax and hypomethylating agents in older/unfit patients with blastic plasmacytoid dendritic cell neoplasm. Am J Hematol. 97:E62–7. DOI: 10.1002/ajh.26417. PMID: 34807470. PMCID: PMC9541522.
47. Samhouri Y, Ursu S, Dutton N, Tanvi V, Fazal S. 2021; Tagraxofusp followed by combined azacitidine and venetoclax in blastic plasmacytoid dendritic cell neoplasm: a case report and literature review. J Oncol Pharm Pract. 27:990–5. DOI: 10.1177/1078155220951850. PMID: 32847479.
48. Sapienza MR, Fuligni F, Agostinelli C, et al. 2014; Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 28:1606–16. DOI: 10.1038/leu.2014.64. PMID: 24504027. PMCID: PMC4294271.
49. Marmouset V, Joris M, Merlusca L, et al. 2019; The lenalidomide/bortezomib/dexamethasone regimen for the treatment of blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol. 37:487–9. DOI: 10.1002/hon.2671. PMID: 31465531.
50. Cytlak U, Resteu A, Bogaert D, et al. 2018; Ikaros family zinc finger 1 regulates dendritic cell development and function in humans. Nat Commun. 9:1239–40. DOI: 10.1038/s41467-018-02977-8. PMID: 29588478. PMCID: PMC5869589. PMID: 052768b869044a2d884f1ad1b5b273c9.
51. Mirgh S, Sharma A, Folbs B, et al. 2021; Daratumumab-based therapy after prior azacytidine-venetoclax in an octagenerian female with BPDCN (blastic plasmacytoid dendritic cell neoplasm ) - a new perspective. Leuk Lymphoma. 62:3039–42. DOI: 10.1080/10428194.2021.1941938. PMID: 34151693.
52. Renosi F, Callanan M, Lefebvre C. 2022; Genetics and epigenetics in neoplasms with plasmacytoid dendritic cells. Cancers (Basel). 14:4132. DOI: 10.3390/cancers14174132. PMID: 36077669. PMCID: PMC9454802. PMID: 190f7a06fe104728896d2e742006a09e.
Table 1
Diagnostic work-up for blastic plasmacytoid dendritic cell neoplasms.
Table 2
Immunophenotypic diagnostic criteria of blastic plasmacytoid dendritic cell neoplasms according to 5th WHO classification.
| Expected positive expression: CD123*, TCF4*, TCL1*, CD303*, CD304*, CD4, CD56 |
| Expected negative markers: CD3, CD14, CD19, CD34, lysozyme, myeloperoxidase |
|
Immunophenotypic diagnostic criteria |
Table 3
Differential diagnosis of hematopoietic malignancies based on skin biopsy.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download