Abstract
Transarterial chemoembolization (TACE) is a widely used hepatocellular carcinoma (HCC) treatment. Some cases of supraumbilical skin rash after TACE in patients with HCC have been reported. To the best of the authors’ knowledge, there are no reports on atypical, generalized rashes caused by doxorubicin systemic absorption after TACE. This paper presents the case of a 64-year-old male with HCC who developed generalized macules and patches one day after a successful TACE procedure. A histology examination of a skin biopsy of a dark reddish patch on the knee revealed severe interface dermatitis. He was treated with a topical steroid, and all skin rashes improved within a week with no side effects. This report presents this rare case with a literature review on skin rash after TACE.
Go to : 
Transarterial chemoembolization (TACE) is the treatment of choice for hepatocellular carcinoma (HCC) when local ablation, resection, or liver transplantation is not possible in patients with the intermediate Barcelona clinic liver cancer (BCLC)-B stage.1 TACE is a treatment option even in the early stages, based on the concept that treatment stage migration is possible when specific patient profiles are considered. On the other hand, TACE is associated with several complications. Nonvascular complications include postembolization syndrome, liver abscess, biloma, biliary strictures, and hepatic failure.2 Vascular complications include a puncture site hematoma, hepatic artery injury, arterial spasm, dissection, or thrombosis.2 Non-target embolization of the left or right hepatic artery, gastroduodenal artery, or cystic artery with chemoembolic agents result in gastrointestinal mucosal ulceration, perforation, pancreatitis, and cholecystitis.2 Localized skin rashes in the abdomen caused by non-target embolization via the hepatic falciform artery (HFA) after TACE are reported infrequently.3-10 To the best of the authors’ knowledge, however, no systemic cutaneous side effects related to doxorubicin systemic absorption after TACE have been reported. This paper presents the first case of an atypical, generalized skin rash after TACE in a patient with HCC.
Go to : 
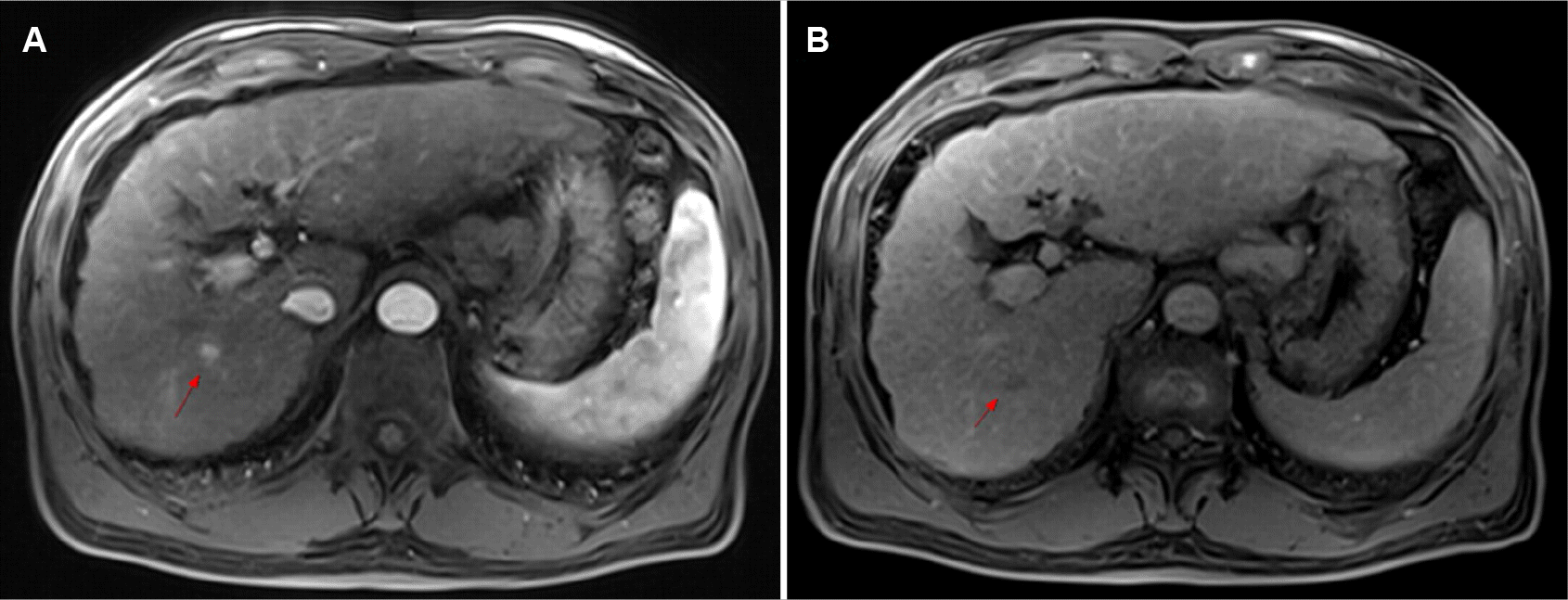
A 64-year-old male has been followed-up in an outpatient setting for liver cirrhosis caused by a chronic viral hepatitis B infection with a preserved liver function (Child–Pugh Stage A) since 2017 in Chonnam National University Hospital, Gwangju, South Korea. In December 2020, a 1.7 cm ill-defined heterogeneously hyperechoic lesion was detected in the right hepatic lobe on ultrasonography (US) for surveillance. His alpha-fetoprotein level was 3.20 ng/mL (reference value: 0.89–8.78 ng/mL), and his protein induced by vitamin K absence- II level was 28 mAU/mL (reference value: 0–40 mAU/mL). All other laboratory tests were within the normal range, including complete blood count with differential, liver function tests, renal function tests, and electrolytes. Magnetic resonance imaging of the liver showed that the mass was 1 cm in size, with enhancement in the arterial phase and low signal intensity in the hepatobiliary phase (Fig. 1). No vascular invasion, thrombosis, metastasis, or enlarged lymph nodes were found. The mass was highly suspicious for HCC and was determined to be BCLC Stage 0 because the patient had a good performance status. Ablation could not be performed because of the poor delineation of the index tumor when planning the US for radiofrequency ablation, and the patient refused a surgical resection or transplantation. Therefore, TACE was performed.
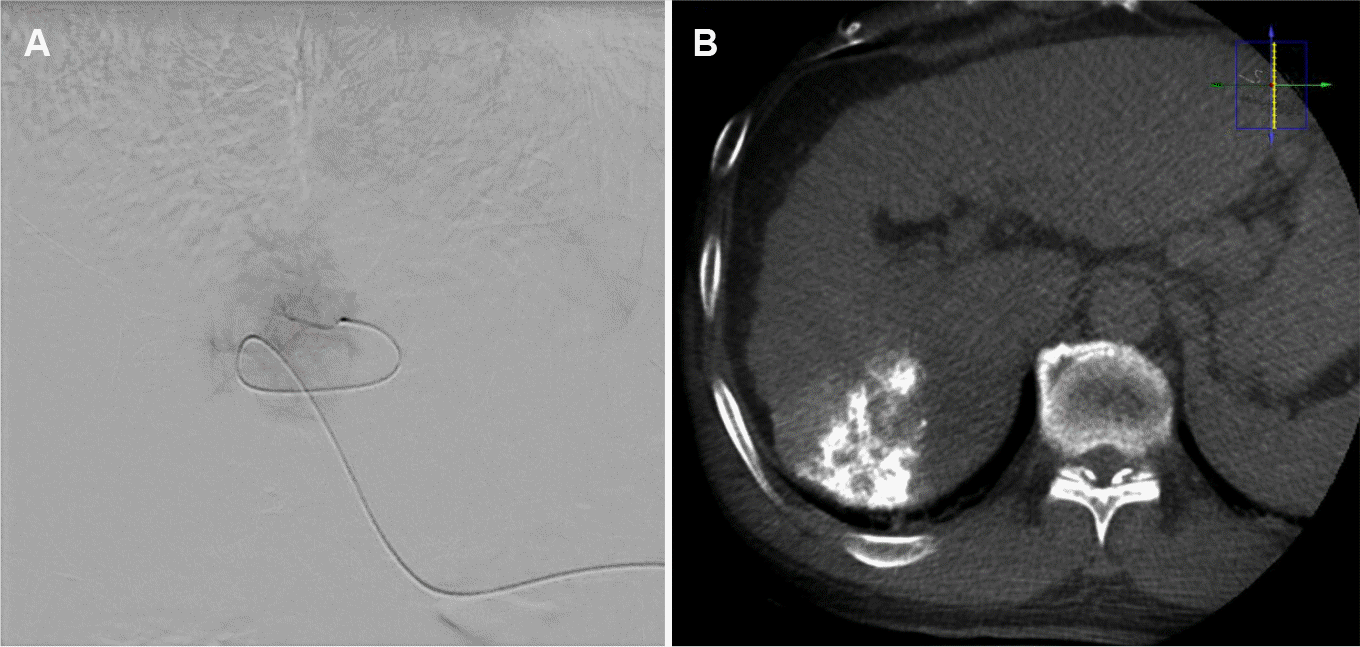
Angiographies of the celiac axis, superior mesenteric artery, and common hepatic artery were performed. The celiac axis was normal without arterioportal or arteriovenous shunts. Selective angiography was performed using a microcatheter to locate the tumor branches in the right hepatic artery, and hypervascular tumoral staining consistent with the target lesion was confirmed in the right hepatic lobe (Fig. 2). The branch was chemoembolized with a mixture of lipiodol (2 mL) and doxorubicin (10 mg). Postembolization C-arm CT and angiography revealed a compact lipiodol uptake lesion in the right hepatic lobe. The patient tolerated the procedure well.
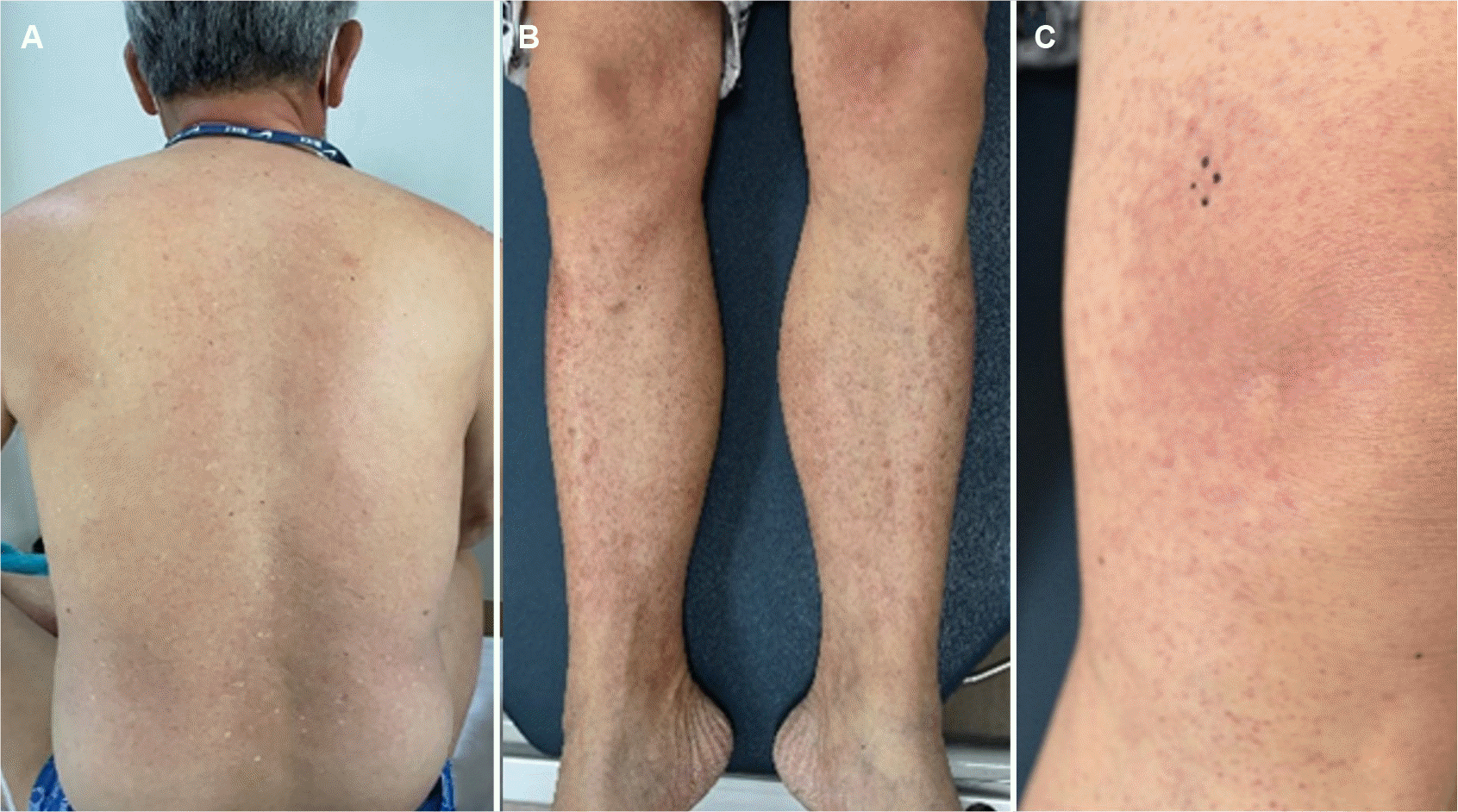
The following day, the patient complained of a generalized skin rash, with multiple purpuric macules and patches of varying sizes on the extremities and quickly spreading to the trunk and neck (Fig. 3). There were no other symptoms, such as pain, fever, or pruritus. The petechia-like macules of the lower extremities were most noticeable, but more reddish patches were observed in the left lower thigh and knee. There was no special medication or known allergy history other than TACE. The contrast agent (Iomeprol, a non-ionic water-soluble contrast medium) used for angiography was the same as that used in previous CT scans, and the patient showed no adverse events, such as allergic reactions. Four days after TACE, the rash on the trunk and neck appeared to fade, but the lesions on the extremities did not improve. Complete blood cell count and differential count, including eosinophil and other blood tests, were consistently normal. Although it was not administered systemically, doxorubicin used during TACE was assumed to be the culprit drug. A topical steroid (methylprednisolone aceponate, Topisol Milk Lotion; Kolon pharmaceutical, Seoul, Korea) was applied to treat the possible doxorubicin-induced hypersensitivity and vasculitis for two weeks. A punch biopsy of the leg rash was also performed.
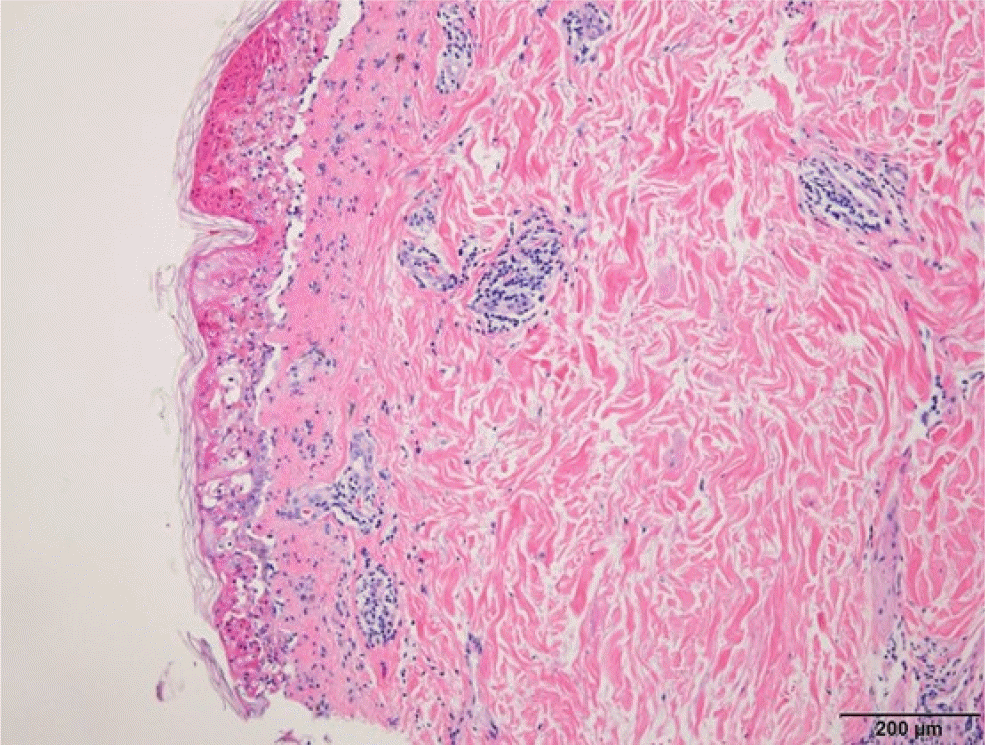
A histological examination of the biopsy specimen revealed full-thickness epidermal necrosis and severe interface dermatitis with subepidermal blister formation, which was consistent with drug eruption, but no definitive evidence of vasculitis was found (Fig. 4). All skin lesions disappeared within a week of using the topical steroid. No sequelae, such as scars or pigmentation, remained. After three weeks, the follow-up CT scan revealed no evidence of HCC recurrence, and there was no allergic reaction to the contrast agent. Written informed consent was obtained from the patient for publication of this case report.
Go to : 
After TACE, various adverse events may occur.11 One of the most common complications following TACE is a postembolization syndrome, which is characterized by fever, abdominal pain, and leukocytosis caused by an inflammatory response to necrotic tissue.11 Other well-known complications after TACE include abdominal pain, inguinal hematoma, hepatic dysfunction, acute cholecystitis, and liver abscess.11 On the other hand, localized skin complications, such as supraumbilical skin rash associated with the HFA after TACE, have been reported infrequently.3,5,6,9 To the best of the authors’ knowledge, no systemic skin toxicity caused by doxorubicin after TACE has been reported.
In the present case, petechiae-like macules and patches appeared on the extremities and trunk the day after TACE and improved one week after topical steroid use. Doxorubicin was considered the culprit drug because no other medical or allergen exposure or adverse reactions were found on multiple CT scans before and after TACE. The doxorubicin-induced hypersensitivity and vasculitis were suspected because most of the skin rash was petechiae, with some focal exanthematous (maculopapular) rash around the left lower thigh and knee. A histopathological examination revealed full-thickness epidermal necrosis and severe interface dermatitis with subepidermal blister formation, indicating exanthematous drug eruption. No red blood cells or vascular damage were found in the histopathologic examination, which is a typical finding in leukocytoclastic vasculitis. A skin biopsy near the left knee revealed more dark reddish patches rather than a petechiae- like rash. This may explain why no leukocytoclastic vasculitis was found in the biopsy specimen. A biopsy of the petechial lesion on the shin area suggested leukocytoclastic vasculitis, but the patient refused to undergo a second skin biopsy.
Skin injury after TACE is a rare but well-known complication. A search of the English literature on PubMed yielded several cases of localized skin rash after TACE.3-10,12-16 In all cases, the skin rashes appeared as brownish or erythematous patches or nodules in the supraumbilical area.3-10,12-16 The skin rashes were painful in most cases,3-7,9 and appeared one day after TACE,4,9,13 but could appear up to five days after the procedure.6,7 A skin biopsy revealed fat necrosis with dermal sclerosis and thrombosis or epidermal separation.4,5,7 Oral non-steroidal anti-inflammatory drugs, pentoxifylline, local steroid injection, and local laser therapy are commonly used to treat patients,3,5,7-9 but a total excision was required in one case.10 The rash and pain disappeared in two weeks, but the pigmentation or subcutaneous nodule remained for a year.3,6,9 The skin rash was caused by a chemotherapeutic agent distribution into the HFA with vaso-occlusion.3,5,6,8-10,12,13
In the present case, the patient also developed a skin rash after TACE. The skin rash, however, appeared on the extremities and trunk, unlike the localized rash in previous reports. Furthermore, the histopathological findings revealed no fat necrosis or thrombosis, which are common in previous reports. Therefore, the skin rash in this patient was not attributed to vaso-occlusion caused by lipiodol or drug-eluting beads. The skin rash was considered a drug eruption caused by systemic doxorubicin absorption through a concealed route.
This report is limited to only one case. On the other hand, as TACE using doxorubicin is an important and widely used treatment for HCC, clinicians must be more vigilant about uncommon side effects. If the skin complication was overlooked, the patient might have experienced great discomfort and suffered from sequelae. An awareness of this complication is important for follow-up and further treatment of HCC in this patient. Therefore, early recognition of rare skin complications of TACE using doxorubicin, together with a pathological examination, CT scan review, and appropriate medical treatment, is essential.
Go to : 
Notes
Financial support
This study was supported by a grant (BCRI22067) of Chonnam National University Hospital Biomedical Research Institute.
Go to : 
REFERENCES
1. Reig M, Forner A, Rimola J, et al. 2022; BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76:681–693. DOI: 10.1016/j.jhep.2021.11.018. PMID: 34801630. PMCID: PMC8866082.
2. Clark TW. 2006; Complications of hepatic chemoembolization. Semin Intervent Radiol. 23:119–125. DOI: 10.1055/s-2006-941442. PMID: 21326755. PMCID: PMC3036366.
3. Kanzaki H, Nouso K, Miyahara K, et al. 2009; A case of hepatocellular carcinoma with skin injury of the upper abdominal wall after transcatheter arterial chemoembolization: a case report. Cases J. 2:7197. DOI: 10.4076/1757-1626-2-7197. PMID: 19918514. PMCID: PMC2769344.
4. Lee E, Kim GM, Kim SY. 2009; Fixed drug eruption mimicking supraumbilical skin rash: one of rare complication of transarterial chemoembolization. J Eur Acad Dermatol Venereol. 23:324–325. DOI: 10.1111/j.1468-3083.2008.02863.x. PMID: 18631209.
5. Byun JW, Han SH, Song HJ, et al. 2009; A case of supraumbilical skin rash after chemoembolization for hepatocellular carcinoma. J Eur Acad Dermatol Venereol. 23:1458–1459. DOI: 10.1111/j.1468-3083.2009.03252.x. PMID: 19453804.
6. Hama Y, Iwasaki Y, Kusano S. 2000; Supraumbilical skin rash after chemoembolization for hepatocellular carcinoma. Eur Radiol. 10:1356. DOI: 10.1007/s003309900297. PMID: 10939508.
7. Jang MS, Baek JW, Kang DY, Kang JS, Suh KS, Kim ST. 2011; Supraumbilical skin rash after transcatheter arterial chemoembolization: successful treatment with pentoxifylline. J Dermatol. 38:1188–1191. DOI: 10.1111/j.1346-8138.2011.01231.x. PMID: 21592200.
8. Nagpal P, Bhalala M, Vidholia A, et al. 2016; Abdominal skin rash after TACE due to non-target embolization of hepatic falciform artery. ACG Case Rep J. 3:217–220. DOI: 10.14309/crj.2016.55. PMID: 27144210. PMCID: PMC4843162.
9. Lin CC, Wu DK, Shih PM, Liu GC, Chuang WL. 2004; Supraumbilical skin rash and fat necrosis after transcatheter arterial chemoembolization: a case report. Kaohsiung J Med Sci. 20:36–40. DOI: 10.1016/S1607-551X(09)70082-8. PMID: 15481565.
10. Kim HY, Bae SH, Park CH, et al. 2013; Supraumbilical subcutaneous fat necrosis after transcatheter arterial chemoembolization with drug-eluting beads: case report and review of the literature. Cardiovasc Intervent Radiol. 36:276–279. DOI: 10.1007/s00270-012-0356-6. PMID: 22382809.
11. Marcacuzco Quinto A, Nutu OA, San Román Manso R, et al. 2018; Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir Esp (Engl Ed). 96:560–567. English, Spanish. DOI: 10.1016/j.cireng.2018.10.017.
12. Umemura T, Yamamura N, Nagata A, et al. 1998; Case report: Steatonecrosis in the upper abdomen following transcatheter arterial embolization for hepatocellular carcinoma. J Gastroenterol Hepatol. 13:471–474. DOI: 10.1111/j.1440-1746.1998.tb00670.x. PMID: 9641642.
13. Brennan DD, Farrelly C, Cooney R, Norris S, McEniff N. 2005; Abdominal rash after transarterial chemoembolization via the right inferior phrenic artery. J Vasc Interv Radiol. 16:1269. DOI: 10.1097/01.RVI.0000179798.81815.60. PMID: 16151071.
14. Grieshaber E, Nicotri T, Reina R, Rupley K, Wang A. 2014; Cutaneous embolization of doxorubicin drug-eluting beads. JAMA Dermatol. 150:1118–1120. DOI: 10.1001/jamadermatol.2014.305. PMID: 24989853.
15. Elsayed AG, Martin JM, Pacioles T. 2018; Rash and subcutaneous fat necrosis after DEB-TACE with doxorubicin. BMJ Case Rep. 2018:bcr2017222394. DOI: 10.1136/bcr-2017-222394. PMID: 29305365. PMCID: PMC5775767.
16. Kuan A, Khoo L, Yang SS, Chia HY, Lee JSS. 2020; Retiform purpura as a complication of microsphere emboli following transarterial chemoembolization for primary hepatocellular carcinoma: A case report and literature review. Am J Dermatopathol. 42:e108–e110. DOI: 10.1097/DAD.0000000000001586. PMID: 31809275.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download