Abstract
Background/Aims
To investigate the risk of metabolic syndrome and fatty liver diseases in gastric cancer survivors compared to non-cancer subjects.
Methods
The data from the health screening registry of the Gangnam Severance Hospital from 2014–2019 was used. Ninety-one gastric cancer survivors and a propensity-score-matching 445 non-cancer subjects were analyzed. Gastric cancer survivors were divided into those with surgical treatment (OpGC, n=66) and non-surgical treatment (non-OpGC, n=25). Metabolic syndrome, fatty liver by ultrasonography, and metabolic dysfunction-associated fatty liver disease (MAFLD) were assessed.
Results
Metabolic syndrome was in 15.4% of gastric cancer survivors (OpGC; 13.6%, non-OpGC; 20.0%). Fatty liver by ultrasonography was in 35.2% in gastric cancer survivors (OpGC; 30.3%, non-OpGC: 48.0%). MAFLD was in 27.5% of gastric cancer survivor (OpGC; 21.2%, non-OpGC; 44.0%). After adjusting for age, sex, smoking, and alcohol, the risk of metabolic syndrome was lower in OpGC than in non-cancer subjects (OR, 0.372; 95% CI, 0.176-0.786, p=0.010). After adjusting, OpGC showed lower risks of fatty liver by ultrasonography (OR, 0.545; 95% CI, 0.306-0.970, p=0.039) and MAFLD (OR, 0.375; 95% CI, 0.197-0.711, p=0.003) than did non-cancer subjects. There were no significant differences in the risks of metabolic syndrome and fatty liver diseases between non-OpGC and non-cancer subjects.
Conclusions
OpGC showed lower risks of metabolic syndrome, fatty liver by ultrasonography, and MAFLD than non-cancer subjects, but there were no significant differences in the risks between non-OpGC and non-cancer subjects. Further studies on metabolic syndrome and fatty liver diseases in gastric cancer survivors are warranted.
The number of cancer survivors is increasing rapidly with the recent developments in cancer treatments. Cancer survivors comprise a newly formed patient population that needs comprehensive care.1 Cancer survivors have a lifetime risk of high morbidity and mortality and are vulnerable to second-primary cancers, requiring them to be educated about healthy behaviors.2 Therefore, evaluating and managing accompanied comorbidities and survival have become important issues in cancer survivors.3
Clinicians need to understand these issues in cancer survivors to develop follow-up care plans that allow for adequate surveillance, prevention, and management of long-term and late effects of cancer. Comprehensive care is key to developing a cancer control plan in public health.1
In Korea, gastric cancer is the second most common cancer in men.4 A previous study reported that the odds of metabolic syndrome for cancer survivors might differ by cancer type and sex.5 The prevalence of each metabolic syndrome component was significantly higher in cancer survivors than in controls, with a higher prevalence in colorectal, breast, cervical, lung, thyroid, prostate, and bladder cancer survivors but not in gastric and liver cancer survivors.6 Moreover, the fatty liver increases the risk of all-cause and cancer-specific mortality in cancer survivors.7 Recently, international experts proposed redefining non-alcoholic fatty liver disease (NAFLD) as metabolic dysfunction-associated fatty liver disease (MAFLD) based on modified criteria.8
This study compared the prevalence and risk of metabolic syndrome and fatty liver diseases in gastric cancer survivors with healthy controls. Metabolic syndrome, fatty liver by ultrasonography, and MAFLD were assessed in gastric cancer survivors with surgical treatment and gastric cancer survivors with non-surgical treatment compared with non-cancer subjects.
The data from a health check-up screening registry of 17,497 participants who visited the Gangnam Severance Hospital from 2014 to 2019 for a health check-up were used. The participants were offered health-screening examinations by their employers or voluntarily participated in health evaluation follow-up programs. The information obtained in the questionnaire included the frequency of alcohol consumption per week and the average amount of alcohol consumed at once.
Studies on gastric cancer survivors presented different criteria for gastric cancer survivors.9-12 Patients were considered gastric cancer survivors if they satisfied all the following criteria: (1) patients who had cancer in the past, (2) had completed initial cancer management, and (3) had no apparent evidence of recurrence for more than three years for patients who underwent endoscopic treatment or non-surgical treatment and for more than five years for patients who survived surgical treatment. Non-cancer subjects were individuals who had not been diagnosed with gastric cancer.
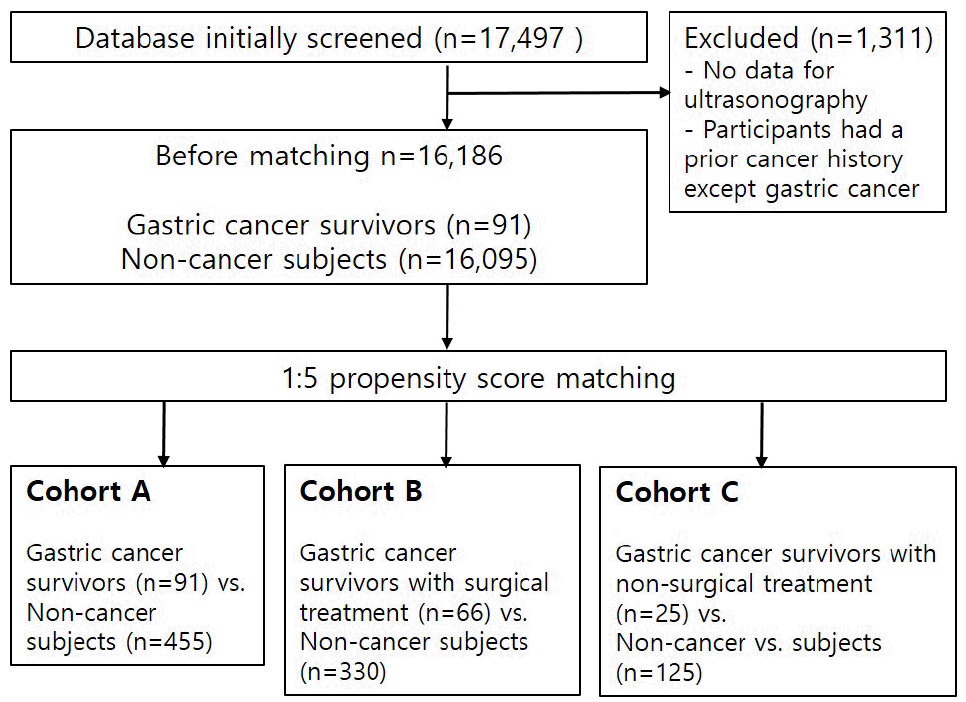
The exclusion criteria were (1) absent ultrasound findings and (2) participants with a prior cancer history except for gastric cancer. After exclusion, 16,186 participants were enrolled and 91 gastric cancer survivors were identified. The gastric cancer survivors were divided into those with surgical treatment (OpGC, n=66) and non-surgical treatment (non-OpGC, n=25). Among OpGC, 56 had undergone a subtotal gastrectomy and 10 had undergone a total gastrectomy. Age, sex, smoking, and alcohol 1:5 propensity matching was performed each for gastric cancer survivors with non-cancer survivors, OpGC with non-cancer subjects, and non-OpGC with non-cancer subjects: (1)Cohort A, 1:5 propensity matching for gastric cancer survivors (n=91) and non-cancer subjects (n=455); (2) Cohort B, 1:5 propensity matching for OpGC (n=66) and non-cancer subjects (n=330); (3) Cohort C, 1:5 propensity matching for non-OpGC (n=25) and non-cancer subjects (n=125). Three times 1:5 propensity score matching was done for analysis. Fig. 1 shows a flowchart of study enrollment. The study protocol was approved by the Institutional Review Board of the Gangnam Severance Hospital exams (IRB No. 3-2019-0076), which waived the requirement for informed consent as de-identified data obtained from a health screening registry was used. The participants signed a comprehensive agreement that allowed the use of their data for medical studies following de-identification.
The participants’ demographics and medical history were collected retrospectively by reviewing medical records. The former included age, sex, height, weight, BMI, waist circumference, and blood pressure (BP), which were measured at the time of each evaluation. The past medical history was collected from a self-administered medical questionnaire. Hypertension was defined as a systolic BP ≥140 mmHg, a diastolic BP ≥90 mmHg, and the current use of antihypertensive agents. Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dL and the current use of anti-diabetic medications. Current smokers were classified as smokers, whereas ex-smokers were identified as non-smokers. The laboratory evaluation included measurements of the total cholesterol, triglyceride, LDL, HDL, and fasting glucose levels.
Metabolic syndrome was diagnosed in participants according to the guidelines of the Korean Academy of Family Medicine.13 The guideline defined metabolic syndrome traits as abdominal obesity, high triglyceride level, reduced HDL cholesterol, increased BP, and an elevated fasting blood sugar level. Abdominal obesity was defined as a waistline of at least 85 cm for women and 90 cm for men. High triglyceride levels are ≥150 mg/dL or ≥1.7 mmol/L in blood. Reduced HDL-cholesterol levels were defined as a serum level <40 mg/dL (1.04 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women. BP was considered elevated if it was ≥130/85 mmHg or medication for hypertension was used. The fasting blood sugar levels were considered elevated if they were ≥100 mg/dL (5.6 mmol/L). The patients were considered as having metabolic syndrome if they had three or more of the above-mentioned traits.
A fatty liver was assessed by ultrasonography. Abdominal ultrasonography was performed by skilled radiologists who were blinded to the clinical and laboratory data of the study subjects at the time of examination. A fatty liver was defined as the presence of at least two of these ultrasonographic features: (1) a diffuse increase in the fine echoes of the liver parenchyma compared with the kidney or spleen, (2) an ultrasound beam attenuation, and (3) poorly visualized intra-hepatic structures.14 Each fatty liver feature was scored. A score of 2 indicated a definite positive: 1, a probable positive; and 0, a negative. The total score of fatty liver ranged from 0–6, where a total score of 1–2 indicated mild fat infiltration; a score of 3–4 indicated moderate infiltration; a total score of 5–6 indicated severe fat infiltration. A score of 0 indicated the absence of fatty liver.15
According to the recently proposed diagnostic criteria,8 MAFLD was diagnosed based on the presence of hepatic steatosis (i.e., fatty liver by ultrasonography), in addition to obesity or overweight (BMI ≥23 kg/m2), type 2 diabetes mellitus, or evidence of at least two metabolic risk abnormalities in lean or normal weight patients, which are as follows: (1) waist circumference ≥90 cm in Asian men and ≥80 cm in Asian women; (2) BP ≥130/85 mmHg or specific drug treatment; (3) plasma triglycerides ≥150 mg/dL or specific drug treatment; (4) plasma HDL-cholesterol <40 mg/dL for men and <50 mg/dL for women or specific drug treatment; (5) prediabetes, including fasting glucose levels 100–125 mg/dL or 2-h post-load glucose levels of 140–199 mg/dL.16
The baseline characteristics were expressed as frequencies and percentages for categorical variables and mean±standard deviations for continuous variables. The Student’s t-test, χ2 test, and analysis of variance were used to compare variables. A multivariable-adjusted logistic regression analysis was used to determine the risk ratio of metabolic syndrome, fatty liver by ultrasonography, and MAFLD according to the relevant variables. All statistical tests were two-tailed, and a p-value <0.05 was considered statistically significant. All statistical analyses were performed by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Table 1 lists the baseline demographic characteristics. The mean age of gastric cancer survivors was 60.1±10.0 years, and the cancer-free interval of gastric cancer survivors was 11.4±7.5 years. The prevalence of metabolic syndrome was lower in gastric cancer survivors than in non-cancer subjects (15.4% vs. 28.8%, p=0.008) and was also lower in OpGC than in non-cancer subjects (13.6% vs. 29.4%, p=0.008), but there was no significant difference in the prevalence of metabolic syndrome between non-OpGC and non-cancer subjects (20.0% vs. 27.2%, p=0.454).
Among the metabolic syndrome components, elevated blood pressure (19.7% vs. 32.4%, p=0.040), abdominal obesity (12.1% vs. 32.4%, p=0.001), and low HDL cholesterol (12.1% vs. 31.2%, p=0.002) were lower in OpGC than in non-cancer subjects. There were no differences in the components of metabolic syndrome between non-OpGC and non-cancer subjects. The mean count of metabolic syndrome components was lower in OpGC than in the non-cancer subjects (1.1±1.1 vs. 1.8±1.3, p<0.001). There was no significant difference in the mean count of metabolic syndrome components between non-OpGC and non-cancer subjects.
The difference in the prevalence of fatty liver by ultrasonography was significantly lower in OpGC than in the non-cancer subjects (30.3% vs.43.9%, p=0.040) but no significant difference between non-OpGC and non-cancer subjects. Mild-grade fatty liver was the most common among each group with fatty liver. MAFLD was less prevalent in OpGC than in the non-cancer subjects (21.2% vs. 40.9%, p=0.003). There was no significant difference in MAFLD between non-OpGC and non-cancer subjects.
Compared to non-cancer subjects, gastric cancer survivors showed a lower risk of metabolic syndrome (OR, 0.450; 95% CI, 0.246–0.823, p=0.010). After adjusting for age, sex, smoking, and alcohol, the risk of metabolic syndrome was significantly lower in gastric cancer survivors (OR, 0.444; 95% CI, 0.242–0.817, p=0.009) (Table 2). After adjusting, OpGC showed a lower risk of metabolic syndrome than non-cancer subjects (OR, 0.372; 95% CI, 0.176–0.786, p=0.010). There was no significant difference in the risk of metabolic syndrome between non-OpGC and non-cancer subjects (OR, 0.673; 95% CI, 0.229–1.974, p=0.470).
After adjusting for age, sex, smoking, and alcohol, OpGC with a history of subtotal gastrectomy showed a lower risk of metabolic syndrome than non-cancer subjects (OR, 0.403; 95% CI, 0.182–0.888, p=0.024) (Supplementary Table 1). Considering the methods of anastomosis, OpGC with a history of Billroth II anastomosis of subtotal gastrectomy showed a lower risk of metabolic syndrome than non-cancer subjects (OR, 0.145; 95% CI, 0.026–0.800, p=0.027).
Compared with non-cancer subjects, OpGC showed a lower risk of fatty liver by ultrasonography (OR, 0.555; 95% CI, 0.314–0.979, p=0.042). After adjusting for age, sex, smoking, and alcohol, OpGC showed a lower risk of fatty liver by ultrasonography than the non-cancer subjects (OR, 0.545; 95% CI, 0.306–0.970, p=0.039) (Table 3). There was no significant difference in the risk of fatty liver by ultrasonography between non-OpGC and non-cancer subjects.
Compared to non-cancer subjects, gastric cancer survivors showed a lower risk of MAFLD (OR, 0.590; 95% CI, 0.359– 0.969, p=0.037). After adjusting for age, sex, smoking, and alcohol, the risk of MAFLD was significantly lower in the gastric cancer survivors (OR, 0.570; 95% CI, 0.344–0.946, p=0.030) (Table 4). After adjusting, OpGC showed a lower risk of MAFLD than the non-cancer subjects (OR, 0.375; 95% CI, 0.197– 0.711; p=0.003). There was no significant difference in the risk of MAFLD between non-OpGC and non-cancer subjects.
After adjusting for age, sex, smoking, and alcohol, OpGC with subtotal gastrectomy showed a lower risk of MAFLD than the non-cancer subjects (OR, 0.435; 95% CI, 0.222–0.851, p=0.015) (Supplementary Table 2). Considering the anastomosis methods, there were no significant differences among anastomosis methods in gastric cancer survivors with a history of surgical treatment.
The concerns regarding the long-term health problems of cancer survivors are being increasingly recognized. Various health problems can occur among cancer survivors. In this study, gastric cancer survivors with surgical treatment showed a significantly lower risk of metabolic syndrome, fatty liver by ultrasonography, and MAFLD than non-cancer subjects. There was no significant difference in metabolic syndrome and fatty liver diseases between gastric cancer survivors with non-surgical treatment and non-cancer subjects.
In a previous study, the risk of metabolic syndrome was lower in patients with gastric cancer than in the general population.17 In a meta-analysis, the ORs of metabolic syndrome among cancer survivors were higher than the general population in seven studies but lower in the two studies.18 The risk of metabolic syndrome among overall cancer survivors was reported to be 0.7–4.4 times that among the general population.18 In particular, the prevalence of metabolic syndrome has been demonstrably higher among hematologic malignancy and testicular cancer survivors receiving chemotherapy,19 radiotherapy,20 or stem cell transplantation.21-23 In this study, the risk of metabolic syndrome was lower in the gastric cancer survivors than in the non-cancer subjects. This result is comparable to the results of a South Korean study that showed that gastric cancer survivors had a lower risk of metabolic syndrome than non-cancer subjects.5 In a study from China, early gastric cancer survivors who had undergone subtotal gastrectomy had a lower prevalence of metabolic syndrome and type 2 diabetes mellitus than non-cancer subjects.24 In this study, OpGC with a history of subtotal gastrectomy showed a lower risk of metabolic syndrome than the non-cancer subjects. Moreover, considering the methods of surgical treatment, OpGC, with a history of Billroth II anastomosis of subtotal gastrectomy, showed a lower risk of metabolic syndrome compared to non-cancer subjects. A previous study suggested a lower risk of metabolic syndrome would likely result from gastric surgery.5 Significant improvements in liver fibrosis and steatosis were found in obese people who had undergone sleeve gastrectomy (Bariatric surgery) to reduce body weight.25 In another study from Spain, hepatic function and histological features of the non-alcoholic fatty liver disease improved after laparoscopic sleeve gastrectomy among obese patients.26 Ghrelin and leptin have been considered as circulating hormones that induce appetite and feeding behaviors; the plasma levels of these hormones are decreased in patients who had undergone sleeve gastrectomy.27-30 Reduced ghrelin and leptin levels in gastric cancer survivors who had undergone surgical treatment may have contributed to improvements in metabolic syndrome. The physiological conditions and environment of gastric cancer survivors differ from those of obese people. On the other hand, bariatric surgery and gastrectomy for gastric cancer survivors share analogical anatomical traits with respect to anatomy and surgical techniques. The mechanisms underlying the association between metabolic syndrome and surgery for gastric cancer survivors have not yet been fully elucidated.
In a previous cross-sectional study of 140,000 Korean participants in a health screening program, the reported prevalence rate of NAFLD was 25.2%.31 The reported incidence of NAFLD after gastrectomy for gastric cancer survivors is 5.4%.32 In this study, the prevalence of fatty liver by ultrasonography was significantly lower in OpGC than in non-cancer subjects, but there was no significant difference in the prevalence of fatty liver between non-OpGC and non-cancer subjects. The risk of fatty liver was significantly low in OpGC than in non-cancer subjects. Recently, an international expert consensus statement recommended an updated definition of MAFLD to be used instead of NAFLD.8,16 MAFLD may reflect the current knowledge of fatty liver diseases associated with metabolic dysfunction more accurately than NAFLD.8 In this study, the risk of MAFLD was lower in OpGC, and considering methods of surgical treatments, gastric cancer survivors with subtotal gastrectomy showed a lower risk of MAFLD than did in non-cancer subjects. On the other hand, there was no significant difference between non-OpGC and non-cancer subjects. Regarding risk differences in metabolic syndrome and fatty liver diseases in OpGC and non-OpGC, different strategies might be needed for the management of long-term health problems of gastric cancer survivors according to treatment methods. This study had several limitations. First, this was a cross-sectional study. Therefore, changes in anthropometric data and fatty liver by ultrasonography were not serially checked. Second, it was unclear if gastric cancer survivors had fatty liver by ultrasonography before or after cancer diagnosis. Third, this study was unable to secure a sufficient sample size of gastric cancer survivors who had undergone total gastrectomy and those without a history of surgery. Fourth, the plasma levels of hormones associated with metabolic syndrome, such as ghrelin, leptin, and adiponectin were not checked.
In conclusion, gastric cancer survivors with a history of surgical treatment showed lower risks of metabolic syndrome, fatty liver by ultrasonography, and MAFLD than non-cancer subjects. The differences in the risk of metabolic syndrome and fatty liver diseases between non-OpGC and non-cancer subjects were insignificant. Further studies on metabolic syndrome and fatty liver diseases in gastric cancer survivors are warranted to provide proper healthcare for gastric cancer survivors.
Supplementary material is available at the Korean Journal of Gastroenterology website (https://www.kjg.or.kr/).
REFERENCES
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. 2020; Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 52:335–350. DOI: 10.4143/crt.2020.206. PMID: 32178489. PMCID: PMC7176962.
2. Shin DW, Cho B, Kim SY, Jung JH, Park JH. 2013; Management of cancer survivors in clinical and public health perspectives: current status and future challenges in Korea. J Korean Med Sci. 28:651–657. DOI: 10.3346/jkms.2013.28.5.651. PMID: 23678254. PMCID: PMC3653075.
3. Leach CR, Weaver KE, Aziz NM, et al. 2015; The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 9:239–251. DOI: 10.1007/s11764-014-0403-1. PMID: 25319681.
4. Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. 2020; Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 52:351–358. DOI: 10.4143/crt.2020.203. PMID: 32178488. PMCID: PMC7176954.
5. Shin JY, Choi YH, Song YM. 2015; Metabolic syndrome in Korean cancer survivors and family members: A study in a health promotion center. Nutr Cancer. 67:1075–1082. DOI: 10.1080/01635581.2015.1073752. PMID: 26317444.
6. Kim M, Kim IH, Lim MK, Kim Y, Park B. 2019; Increased prevalence of metabolic syndrome in adult cancer survivors: Asian first report in community setting. Cancer Epidemiol. 58:130–136. DOI: 10.1016/j.canep.2018.12.006. PMID: 30576983.
7. Brown JC, Harhay MO, Harhay MN. 2017; Nonalcoholic fatty liver disease and mortality among cancer survivors. Cancer Epidemiol. 48:104–109. DOI: 10.1016/j.canep.2017.04.007. PMID: 28460349. PMCID: PMC5549274.
8. Eslam M, Sanyal AJ, George J. International Consensus Panel. 2020; MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 158:1999–2014.e1. DOI: 10.1053/j.gastro.2019.11.312. PMID: 32044314.
9. Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, Clarke CA. 2012; Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol. 30:3507–3515. DOI: 10.1200/JCO.2011.35.8028. PMID: 22949151.
10. Park W, Lee JK, Kim CR, Shin JY. 2015; Factors associated with fatigue in Korean gastric cancer survivors. Korean J Fam Med. 36:328–334. DOI: 10.4082/kjfm.2015.36.6.328. PMID: 26634101. PMCID: PMC4666870.
11. Lee JE, Shin DW, Lee H, et al. 2016; One-year experience managing a cancer survivorship clinic using a shared-care model for gastric cancer survivors in Korea. J Korean Med Sci. 31:859–865. DOI: 10.3346/jkms.2016.31.6.859. PMID: 27247493. PMCID: PMC4853663.
12. Kim M, Choi KS, Suh M, Jun JK, Chuck KW, Park B. 2018; Risky Lifestyle behaviors among gastric cancer survivors compared with matched non-cancer controls: Results from baseline result of community based cohort study. Cancer Res Treat. 50:738–747. DOI: 10.4143/crt.2017.129. PMID: 28745037. PMCID: PMC6056967.
13. Shim JY, Kang HT, Kim SY, et al. 2015; Clinical practice guideline of prevention and treatment for metabolic syndrome. Korean J Fam Pract. 5:375–420.
14. Mathiesen UL, Franzén LE, Aselius H, et al. 2002; Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 34:516–522. DOI: 10.1016/S1590-8658(02)80111-6. PMID: 12236486.
15. Chiloiro M, Caruso MG, Cisternino AM, et al. 2013; Ultrasound evaluation and correlates of fatty liver disease: a population study in a Mediterranean area. Metab Syndr Relat Disord. 11:349–358. DOI: 10.1089/met.2012.0169. PMID: 23758075.
16. Eslam M, Newsome PN, Sarin SK, et al. 2020; A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 73:202–209. DOI: 10.1016/j.jhep.2020.03.039. PMID: 32278004.
17. Lee JY, Park NH, Song YS, et al. 2013; Prevalence of the metabolic syndrome and associated factors in korean cancer survivors. Asian Pac J Cancer Prev. 14:1773–1780. DOI: 10.7314/APJCP.2013.14.3.1773. PMID: 23679272.
18. Jung HS, Myung SK, Kim BS, Seo HG. 2012; Metabolic syndrome in adult cancer survivors: a meta-analysis. Diabetes Res Clin Pract. 95:275–282. DOI: 10.1016/j.diabres.2011.08.029. PMID: 22078073.
19. Nuver J, Smit AJ, Wolffenbuttel BH, et al. 2005; The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 23:3718–3725. DOI: 10.1200/JCO.2005.02.176. PMID: 15738540.
20. Barnea D, Raghunathan N, Friedman DN, Tonorezos ES. 2015; Obesity and metabolic disease after childhood cancer. Oncology (Williston Park). 29:849–855.
21. Majhail NS, Flowers ME, Ness KK, et al. 2009; High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 43:49–54. DOI: 10.1038/bmt.2008.263. PMID: 18724397. PMCID: PMC2628412.
22. Annaloro C, Usardi P, Airaghi L, et al. 2008; Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 41:797–804. DOI: 10.1038/sj.bmt.1705972. PMID: 18195686.
23. de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. 2010; The metabolic syndrome in cancer survivors. Lancet Oncol. 11:193–203. DOI: 10.1016/S1470-2045(09)70287-6. PMID: 20152771.
24. Lin XH, Huang KH, Chuang WH, et al. 2018; The long term effect of metabolic profile and microbiota status in early gastric cancer patients after subtotal gastrectomy. PLoS One. 13:e0206930. DOI: 10.1371/journal.pone.0206930. PMID: 30395589. PMCID: PMC6218198.
25. Batman B, Altun H, Simsek B, Aslan E, Namli Koc S. 2019; The effect of laparoscopic sleeve gastrectomy on nonalcoholic fatty liver disease. Surg Laparosc Endosc Percutan Tech. 29:509–512. DOI: 10.1097/SLE.0000000000000672. PMID: 31107849.
26. Cabré N, Luciano-Mateo F, Fernández-Arroyo S, et al. 2019; Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism. 99:81–89. DOI: 10.1016/j.metabol.2019.07.002. PMID: 31279739.
27. Salman MA, El-Ghobary M, Soliman A, et al. 2020; Long-term changes in leptin, chemerin, and ghrelin levels following Roux-en-Y Gastric bypass and laparoscopic sleeve gastrectomy. Obes Surg. 30:1052–1060. DOI: 10.1007/s11695-019-04254-z. PMID: 31713147.
28. Cummings DE. 2006; Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 89:71–84. DOI: 10.1016/j.physbeh.2006.05.022. PMID: 16859720.
29. Klok MD, Jakobsdottir S, Drent ML. 2007; The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 8:21–34. DOI: 10.1111/j.1467-789X.2006.00270.x. PMID: 17212793.
30. Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. 2019; Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 15:502–511. DOI: 10.1016/j.soard.2018.12.002. PMID: 30683512.
31. Jeong EH, Jun DW, Cho YK, et al. 2013; Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol. 19:266–272. DOI: 10.3350/cmh.2013.19.3.266. PMID: 24133664. PMCID: PMC3796676.
32. Kouzu K, Tsujimoto H, Nishikawa M, et al. 2020; Risk factors for nonalcoholic fatty liver disease after gastrectomy for gastric cancer. Gastric Cancer. 23:356–362. DOI: 10.1007/s10120-019-01009-8. PMID: 31555950.
Table 1
Baseline Demographic Characteristics of Gastric Cancer Survivors and Non-cancer Subjects after Propensity Matching
Table 2
Risk of Metabolic Syndrome in Gastric Cancer Survivors Compared with Non-cancer Subjects in Propensity Score-matched Analysis
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.023 (1.003–1.043) | 0.024 | 1.022 (0.999–1.045) | 0.064 | 1.027 (0.984–1.071) | 0.228 |
| Male sex | 1.363 (0.867–2.142) | 0.180 | 1.522 (0.909–2.549) | 0.110 | 1.286 (0.324–5.100) | 0.720 |
| Gastric cancer survivorsa | 0.444 (0.242–0.817) | 0.009 | ||||
| Gastric cancer survivors with surgical treatmenta | 0.372 (0.176–0.786) | 0.010 | ||||
| Gastric cancer survivors with non-surgical treatmenta | 0.673 (0.229–1.974) | 0.470 | ||||
Table 3
Risk of Fatty Liver by Ultrasonography in Gastric Cancer Survivors Compared with Non-cancer Subjects in Propensity Score-matched Analysis
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.985 (0.968–1.003) | 0.104 | 0.996 (0.976–1.017) | 0.734 | 0.968 (0.929–1.008) | 0.111 |
| Male sex | 2.247 (1.498–3.369) | <0.001 | 2.051 (1.303–3.230) | 0.002 | 2.462 (0.733–8.269) | 0.145 |
| Gastric cancer survivorsa | 0.706 (0.438–1.138) | 0.153 | ||||
| Gastric cancer survivors with surgical treatmenta | 0.545 (0.306–0.970) | 0.039 | ||||
| Gastric cancer survivors with non-surgical treatmenta | 1.237 (0.505–3.034) | 0.642 | ||||
Table 4
Risk of Metabolic Dysfunction-associated Fatty Liver Disease in Gastric Cancer Survivors Compared with Non-cancer Subjects in Propensity Score-matched Analysis
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.989 (0.971–1.007) | 0.238 | 0.999 (0.978–1.020) | 0.890 | 0.981 (0.942–1.022) | 0.356 |
| Male sex | 2.500 (1.639–3.814) | <0.001 | 2.505 (1.558–4.029) | 0.0002 | 1.907 (0.565–6.444) | 0.299 |
| Gastric cancer survivorsa | 0.570 (0.344–0.946) | 0.030 | ||||
| Gastric cancer survivors with surgical treatmenta | 0.375 (0.197–0.711) | 0.003 | ||||
| Gastric cancer survivors with non-surgical treatmenta | 1.337 (0.546–3.272) | 0.525 | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download