This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Liver transplant (LT) recipients were considered a vulnerable population during the coronavirus disease 2019 (COVID-19) pandemic. The clinical efficacy of the COVID-19 vaccine is unknown in immunocompromised patients. The purpose of this study was to provide evidence of antibody responses after COVID-19 vaccination in LT recipients.
Methods
This study enrolled 46 patients who underwent LT at Samsung Medical Center (Seoul, Korea) before implementation of the one-dose vaccine in Korea. Those who completed the two-dose COVID-19 vaccine between August 2021 and September 2021 were included and followed through December 2021. Semiquantitative anti-spike serologic testing was performed using the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (Roche Diagnostics, Rotkereuz, Switzerland) with a positive cutoff of at least 0.8 U/mL.
Results
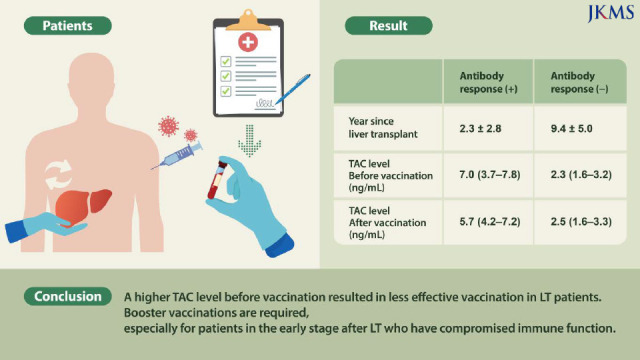
Among all 46 participants, 40 (87%) demonstrated an antibody response after the second dose of a COVID-19 vaccine, while six (13%) had no antibody response after the second dose. Upon univariate analysis, patients with higher antibody titer had longer years since LT (2.3 ± 2.8 vs. 9.4 ± 5.0, P < 0.001). A lower median tacrolimus (TAC) level before vaccination and after the second dose of COVID-19 vaccine indicated a significantly higher antibody response (2.3 [1.6–3.2] vs. 7.0 [3.7–7.8], P = 0.006, 2.5 [1.6–3.3] vs. 5.7 [4.2–7.2], P = 0.003). Period between 2nd vaccination and serologic testing was significantly higher in the antibody-response group compared to the no-antibody-response group (30.2 ± 24.0 vs. 65.9 ± 35.0, P = 0.012). A multivariate analysis of antibody responses revealed TAC level before vaccination as a statistically significant factor.
Conclusion
A higher TAC level before vaccination resulted in less effective vaccination in LT patients. Booster vaccinations are required, especially for patients in the early stage after LT who have compromised immune function.
Keywords: COVID-19, Vaccination, Anti-SARS-CoV-2 S Antibody, Liver Transplantation, Tacrolimus
INTRODUCTION
In 2020, coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization; solid organ transplant recipients, including liver transplant (LT) recipients, were considered a vulnerable population. The earlier breakout of Middle East Respiratory Syndrome (MERS), in 2015, showed that people with underlying diseases or immunocompromise had a high MERS-coronavirus infection rate and a poor prognosis.
1 Based on those results, the same were expected to be true for COVID-19.
During the current rapidly progressing pandemic, COVID-19 vaccines have been issued for emergency use authorization. Vaccination is the most effective prevention strategy, but the clinical efficacy of the COVID-19 vaccine in immunocompromised patients is unknown. In 2021, several studies reported that the antibody response increased to 54% in transplant recipients after the second dose.
2 More recently, there have been reports of serological responses after a third or fourth vaccine dose.
34 All residents in Korea aged 12 or older were eligible to receive vaccinations beginning in February 2021, and the second shot has been available since August 2021.
5 At the time of first inoculation, people were not allowed to choose their vaccine type, and the second shot had to be the same type as the previous inoculation. Therefore, the purpose of this study was to analyze the antibody response after the second dose of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine and to determine the clinical characteristics associated with vaccine non-response in LT recipients.
METHODS
In this prospective study, 46 patients who underwent LT at Samsung Medical Center (Seoul, Korea) were enrolled before receiving the first dose of the COVID-19 vaccine in Korea. Those who completed the second dose of the vaccine between August 2021 and September 2021 were included and followed through December 2021. The exclusion criteria were previous exposure to COVID-19 or those who did not complete a standardized vaccination protocol (12 weeks between AstraZeneca doses, four weeks between Moderna doses, and three weeks between Pfizer doses) in Korea. Clinical data and type of vaccine were obtained from the patient’s medical records, and blood samples were collected up to two months after the second vaccination.
All patients were Korean and received semiquantitative anti-spike serologic testing with the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (Roche Diagnostics, Rotkereuz, Switzerland). The patients were stratified into two groups based on postvaccination antibody level (U/mL): < 0.80 U/mL (undetectable) and ≥ 0.80 U/mL (optimal).
The data are displayed as mean ± standard deviation for continuous variables and as number of patients and percentage for categorical variables. The χ2 statistic was used to assess significant difference between categorical variables. Variables that were significant (P < 0.05) in the simple logistic regression model were included in the multiple logistic regression model.
Ethics statement
This study was approved by the Samsung Medical Center Institutional Review Board, and the participants provided informed consent electronically (IRB file number: 2022-03-108).
RESULTS
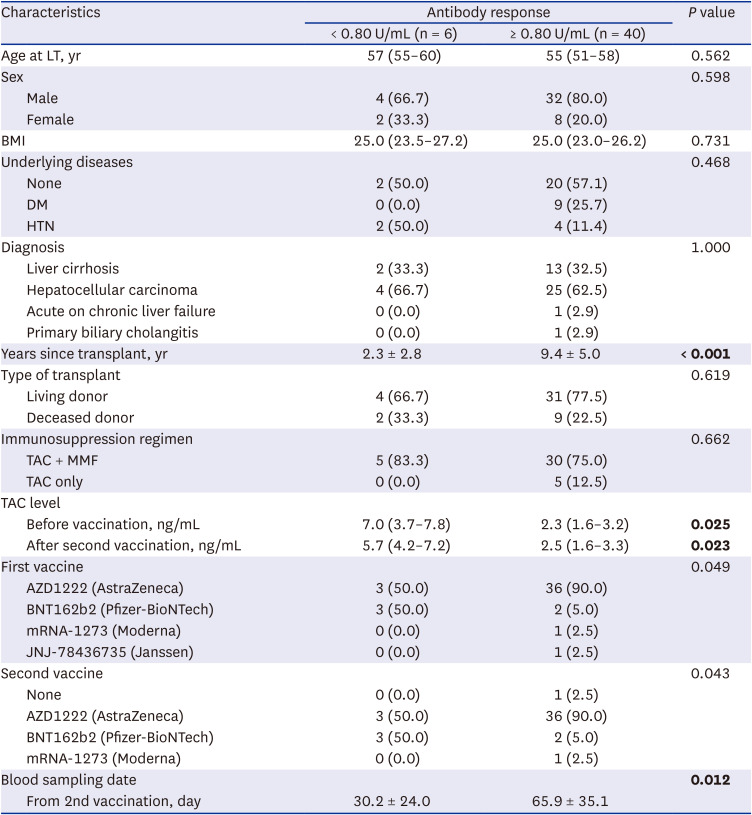
We evaluated 46 transplant recipients who received two doses of the SARS-CoV-2 vaccine (
Table 1); 40 (87%) had a measurable antibody response after the second dose, while six (13%) had no antibody response after the second dose. Patient characteristics are shown in
Table 1. Among the 46 recipients, 39 received the AstraZeneca vaccine, five received the Pfizer vaccine, one received the Moderna vaccine, and one received the Janssen vaccine. No significant differences in sex, body mass index, or etiology of liver disease were noted, and no correlations were identified between the type of transplant and immunosuppression regimen following vaccination.
Table 1
Demographic and clinical characteristics of the participants
|
Characteristics |
Antibody response |
P value |
|
< 0.80 U/mL (n = 6) |
≥ 0.80 U/mL (n = 40) |
|
Age at LT, yr |
57 (55–60) |
55 (51–58) |
0.562 |
|
Sex |
|
|
0.598 |
|
Male |
4 (66.7) |
32 (80.0) |
|
Female |
2 (33.3) |
8 (20.0) |
|
BMI |
25.0 (23.5–27.2) |
25.0 (23.0–26.2) |
0.731 |
|
Underlying diseases |
|
|
0.468 |
|
None |
2 (50.0) |
20 (57.1) |
|
DM |
0 (0.0) |
9 (25.7) |
|
HTN |
2 (50.0) |
4 (11.4) |
|
Diagnosis |
|
|
1.000 |
|
Liver cirrhosis |
2 (33.3) |
13 (32.5) |
|
Hepatocellular carcinoma |
4 (66.7) |
25 (62.5) |
|
Acute on chronic liver failure |
0 (0.0) |
1 (2.9) |
|
Primary biliary cholangitis |
0 (0.0) |
1 (2.9) |
|
Years since transplant, yr |
2.3 ± 2.8 |
9.4 ± 5.0 |
< 0.001
|
|
Type of transplant |
|
|
0.619 |
|
Living donor |
4 (66.7) |
31 (77.5) |
|
Deceased donor |
2 (33.3) |
9 (22.5) |
|
Immunosuppression regimen |
|
|
0.662 |
|
TAC + MMF |
5 (83.3) |
30 (75.0) |
|
TAC only |
0 (0.0) |
5 (12.5) |
|
TAC level |
|
|
|
|
Before vaccination, ng/mL |
7.0 (3.7–7.8) |
2.3 (1.6–3.2) |
0.025
|
|
After second vaccination, ng/mL |
5.7 (4.2–7.2) |
2.5 (1.6–3.3) |
0.023
|
|
First vaccine |
|
|
0.049 |
|
AZD1222 (AstraZeneca) |
3 (50.0) |
36 (90.0) |
|
BNT162b2 (Pfizer-BioNTech) |
3 (50.0) |
2 (5.0) |
|
mRNA-1273 (Moderna) |
0 (0.0) |
1 (2.5) |
|
JNJ-78436735 (Janssen) |
0 (0.0) |
1 (2.5) |
|
Second vaccine |
|
|
0.043 |
|
None |
0 (0.0) |
1 (2.5) |
|
AZD1222 (AstraZeneca) |
3 (50.0) |
36 (90.0) |
|
BNT162b2 (Pfizer-BioNTech) |
3 (50.0) |
2 (5.0) |
|
mRNA-1273 (Moderna) |
0 (0.0) |
1 (2.5) |
|
Blood sampling date |
|
|
0.012
|
|
From 2nd vaccination, day |
30.2 ± 24.0 |
65.9 ± 35.1 |
Of the 46 LT recipients, years since LT was significantly longer in the group with higher antibody titer compared with the no-antibody-response group (2.3 ± 2.8 vs. 9.4 ± 5.0, P < 0.001). Before the first vaccination and after receiving the second vaccination, the median TAC level was significantly higher in the no-antibody-response group compared to the antibody-response group (P = 0.006 and P = 0.003, respectively). A total of 29 patients were diagnosed with liver cancer prior to LT. However, there were no significant differences in antibody response between the liver cancer patients and non-liver cancer patients. Period between 2nd vaccination and serologic testing was significantly higher in the antibody-response group compared to the no-antibody-response group (30.2 ± 24.0 vs. 65.9 ± 35.0, P = 0.012).
Upon multivariable analysis, a factor related significantly to antibody response after the second vaccination in LT recipients was TAC level before vaccination (
Table 2). During the follow-up period, none of the participants had any serious adverse events, graft rejection or experienced COVID-19 infection.
Table 2
Variables associated with factors related to antibody response > 0.8 U/mL after coronavirus disease 2019 vaccination in LT recipients

|
Variables |
Univariate analysis |
Multivariate analysis |
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Male |
0.500 (0.077–3.231) |
0.467 |
- |
- |
|
Age at LT |
1.052 (0.905–1.223) |
0.505 |
- |
- |
|
BMI |
1.076 (0.751–1.541) |
0.689 |
- |
- |
|
DM |
1.667 (0.173–16.023) |
0.658 |
- |
- |
|
HTN |
0.353 (0.052–2.375) |
0.284 |
- |
- |
|
Living donor LT |
1.722 (0.270–10.981) |
0.565 |
- |
- |
|
Years since transplant |
0.540 (0.334–0.873) |
0.012
|
0.639 (0.329–1.242) |
0.639 |
|
TAC level (before vaccination) |
2.527 (1.345–4.748) |
0.004
|
4.705 (1.291–17.151) |
0.019
|
|
TAC level (after 2nd vaccination) |
2.550 (1.328–4.894) |
0.005
|
2.005 (0.156–25.718) |
0.593 |
|
Hepatocellular carcinoma |
1.200 (0.196–7.362) |
0.844 |
- |
- |
DISCUSSION
LT patients are susceptible to infections due to their reduced immunity after surgery, so uncommon viral or fungal infections in the general public are more frequent in LT patients. Six months after LT, immunosuppressants are decreased to a minimum and maintained for the rest of the patient’s life. For this reason, respiratory infections occurring in the community can be especially problematic for these patients. SARS-CoV-2 vaccination has shown an excellent safety profile and significantly reduces the risks of COVID-19 and its complications in immunocompromised patients.
23
In this study, 13% of LT recipients had poor antibody responses to the COVID-19 vaccinations. After adjusting for other variables, TAC level before vaccination was associated with poor antibody responses. Other studies have also described an association between high TAC trough level and a failure to develop detectable SARS-CoV-2 antibody level.
67 In addition, a higher TAC level before vaccination reduces the effectiveness of the vaccine in LT recipients.
Another factor associated with an inferior serological response was the average LT period.
89 Among those who developed antibodies, the average LT period was significantly longer in comparison to that of the lower antibody response group. This finding is consistent with recent results on the effects of COVID-19 antibody responses in patients with a longer duration since transplantation.
67 Our observation suggests that low TAC level and longer period after transplantation may predict and improve LT recipients’ immune response to COVID-19 vaccinations.
No major adverse vaccine or graft rejection events occurred in our cohort. However, we did not measure antibody level after the first dose of the vaccine or after the booster vaccine dose. Other limitations of our study are the inclusion of a small sample of only LT recipients over a short-term follow-up period. Immunocompromised patients are always at risk of opportunistic infections, so research on the need for additional vaccinations according to antibody responses is needed. According to a recent meta-analysis study, LT patients showed a lower seroconversion rate after COVID-19 vaccination compared to patients with chronic liver disease.
8 In addition, the 2021 Korean Advanced Life Support guidelines recommend that LT patients perform the procedure according to medical guidelines and prioritize minimizing the risk of COVID-19 infection.
10
Despite these limitations, we believe our observations are very important and meaningful. The results could help determine the number of vaccines needed. Our results demonstrated that booster vaccines are required, especially for patients in the early stage after LT with compromised immune function.