1. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020; 75(2):285–292. PMID:
31865786.
2. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020; 75(6):1334–1357. PMID:
32370572.
3. Noubiap JJ, Nansseu JR, Nyaga UF, Sime PS, Francis I, Bigna JJ. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019; 105(2):98–105. PMID:
30087099.
4. Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012; 125(13):1594–1596. PMID:
22379111.
5. Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015; 28(3):355–361. PMID:
25156625.
6. Lee KN, Na JO, Choi CU, Lim HE, Kim JW, Kim EJ, et al. Prevalence and characteristics of resistant hypertension at primary clinics in Korea: a nationwide cross-sectional study. Clin Hypertens. 2016; 22(1):4. PMID:
26893938.
7. Smith SM, Gong Y, Handberg E, Messerli FH, Bakris GL, Ahmed A, et al. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014; 32(3):635–643. PMID:
24299915.
8. Kaczmarski KR, Sozio SM, Chen J, Sang Y, Shafi T. Resistant hypertension and cardiovascular disease mortality in the US: results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol. 2019; 20(1):138. PMID:
31023262.
9. Zhao M, Woodward M, Vaartjes I, Millett ER, Klipstein-Grobusch K, Hyun K, et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc. 2020; 9(11):e014742. PMID:
32431190.
10. Brugts JJ, Arima H, Remme W, Bertrand M, Ferrari R, Fox K, et al. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014; 176(3):718–723. PMID:
25189490.
11. Na SH, Lee JH, Lee HY, Lee SY, Kim HS, Chae IH, et al. Risk factors of angiotensin-converting enzyme inhibitor-induced cough in patients with hypertension. Korean Circ J. 2000; 30(12):1540–1545.
12. Os I, Franco V, Kjeldsen SE, Manhem K, Devereux RB, Gerdts E, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008; 51(4):1103–1108. PMID:
18259029.
13. SPRINT Research Group. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015; 373(22):2103–2116. PMID:
26551272.
14. Hermida RC, Crespo JJ, Domínguez-Sardiña M, Otero A, Moyá A, Ríos MT, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020; 41(48):4565–4576. PMID:
31641769.
15. SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021; 42(25):2439–2454. PMID:
34120177.
16. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018; 36(10):1953–2041. PMID:
30234752.
17. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021; 23(3):352–380. PMID:
33605000.
18. Brambilla G, Bombelli M, Seravalle G, Cifkova R, Laurent S, Narkiewicz K, et al. Prevalence and clinical characteristics of patients with true resistant hypertension in central and Eastern Europe: data from the BP-CARE study. J Hypertens. 2013; 31(10):2018–2024. PMID:
23838657.
19. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Crowley K, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013; 34(16):1204–1214. PMID:
23144048.
20. Redfern A, Peters SA, Luo R, Cheng Y, Li C, Wang J, et al. Sex differences in the awareness, treatment, and control of hypertension in China: a systematic review with meta-analyses. Hypertens Res. 2019; 42(2):273–283. PMID:
30518984.
21. Smith SM, Gurka MJ, Calhoun DA, Gong Y, Pepine CJ, Cooper-DeHoff RM. Optimal systolic blood pressure target in resistant and non-resistant hypertension: a pooled analysis of patient-level data from SPRINT and ACCORD. Am J Med. 2018; 131(12):1463–1472.e7. PMID:
30142317.
22. Tsujimoto T, Kajio H. Intensive blood pressure treatment for resistant hypertension. Hypertension. 2019; 73(2):415–423. PMID:
30580680.
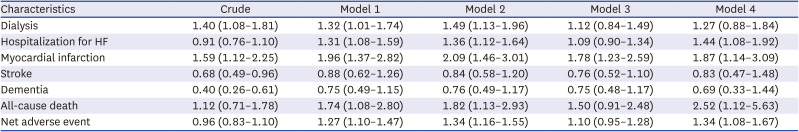
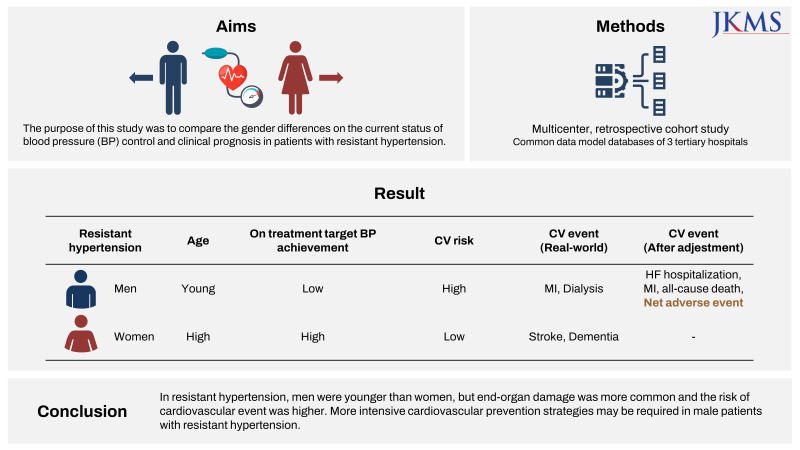
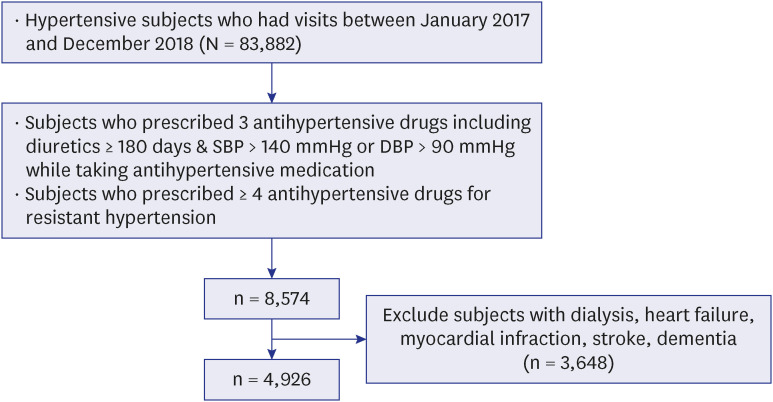
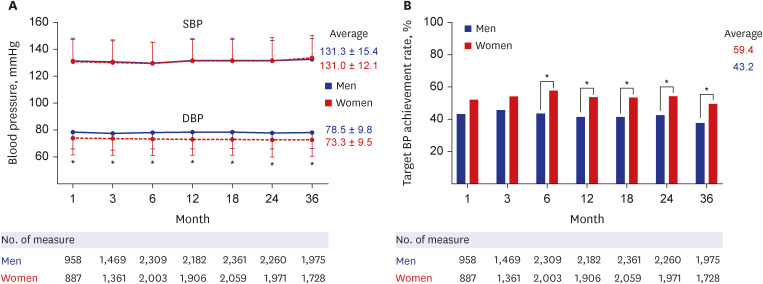
23. Oh JS, Lee CH, Park JI, Park HK, Hwang JK. Hypertension-mediated organ damage and long-term cardiovascular outcomes in Asian hypertensive patients without prior cardiovascular disease. J Korean Med Sci. 2020; 35(48):e400. PMID:
33316856.
24. Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, et al. Resistant hypertension and target organ damage. Hypertens Res. 2013; 36(6):485–491. PMID:
23595044.
25. Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. Prognostic impact of baseline and serial changes in electrocardiographic left ventricular hypertrophy in resistant hypertension. Am Heart J. 2010; 159(5):833–840. PMID:
20435193.
26. Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Italian Society of Hypertension. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012; 26(6):343–349. PMID:
22113443.
27. Papazafiropoulou A, Skliros E, Sotiropoulos A, Papafragos C, Gikas A, Apostolou O, et al. Prevalence of target organ damage in hypertensive subjects attending primary care: C.V.P.C. study (epidemiological cardio-vascular study in primary care). BMC Fam Pract. 2011; 12(1):75. PMID:
21756310.
28. da Costa PM, Cortez AF, de Souza F, Mares GS, Dos Santos BD, Muxfeldt ES. Prognostic impact of baseline urinary albumin excretion rate in patients with resistant hypertension: a prospective cohort study. J Hum Hypertens. 2018; 32(2):139–149. PMID:
29230004.
29. Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. Prognostic importance of baseline and serial changes in microalbuminuria in patients with resistant hypertension. Atherosclerosis. 2011; 216(1):199–204. PMID:
21315356.
30. Salles GF, Cardoso CR, Pereira VS, Fiszman R, Muxfeldt ES. Prognostic significance of a reduced glomerular filtration rate and interaction with microalbuminuria in resistant hypertension: a cohort study. J Hypertens. 2011; 29(10):2014–2023. PMID:
21873887.
31. Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012; 125(13):1635–1642. PMID:
22379110.
32. Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, et al. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014; 64(5):1012–1021. PMID:
25259745.
33. Quan H, Chen G, Walker RL, Wielgosz A, Dai S, Tu K, et al. Incidence, cardiovascular complications and mortality of hypertension by sex and ethnicity. Heart. 2013; 99(10):715–721. PMID:
23403406.
34. Leung AA, Williams JV, Tran KC, Padwal RS. Epidemiology of resistant hypertension in Canada. Can J Cardiol. 2022; 38(5):681–687. PMID:
35122938.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download