CASE REPORT
A 53-year-old woman visited a neurosurgery clinic with left facial swelling, pain, and tenderness. The patient had a history of hepatitis B and hyperlipidemia, and an operative history of the Caldwell-Luc operation 30 years ago due to left sinusitis. Brain magnetic resonance imaging taken at the neurosurgery department showed cystic lesions with low signal intensity and a liquid-liquid level in the left maxillary sinus on T1 images (
Fig. 1A), cystic lesions of high signal intensity with a fluid-fluid level in the left maxillary sinus on T2 images (
Fig. 1B), and rim enhancement on T1-gadolinium enhanced images (
Fig. 1C). She was referred to an otolaryngologist under the suspicion of mucocele, and computed tomography (CT) was performed to identify the lesion. On paranasal sinus CT, a 4.2×3.8 cm hypodense homogenous cystic mass in the left maxillary sinus was observed, with a well-defined margin and bone thinning of the left maxillary sinus wall (
Fig. 2). Based on the patient’s history and imaging tests, we diagnosed the mass as a left postoperative cheek cyst and planned surgical treatment. A cystic mass that bulged out of the left maxillary ostium was identified in the surgical field, and yellow contents flowed out after it was punctured with a suction device. When the maxillary sinus was exposed using a debrider, a pseudocyst consisting of the wall of a cystic mass inside the maxillary sinus was observed on 70° endoscopy. Two days after surgery, there was no evidence of intranasal bleeding and infection. The patient’s pain was well-controlled, and we therefore planned discharge. At the time of discharge, the patient complained of weakness in both lower extremities, but because she was a very thin woman, we assumed that this reflected simple general weakness that appeared after surgery. When she revisited the outpatient department 4 days after surgery, she found it impossible to stand up and walk and squat due to decreased muscle strength in the bilateral lower extremities, and it was difficult for her to use a spoon due to decreased muscle strength in both hands. In order to evaluate neurological symptoms, the department of emergency medicine was consulted. When taking a new history, we additionally confirmed that she had diarrhea for 3 days in the period starting 9 days before surgery. Knee extension and hip flexion were motor grade IV, ankle dorsiflexion was motor grade II, and wrist flexion was motor grade IV. The strength of all other muscles was normal. The Medical Research Council sum score was 48. The Achilles tendon reflex was absent. A cerebrospinal fluid (CSF) test showed no cells, such as white blood cells or red blood cells, and mild albumin cell dissociation was found with an elevated protein of 50 mg/dL. Based on these findings, Intravenous immunoglobulin therapy was administered under the diagnosis of GBS. The patient was admitted to a neurological intensive care unit to control symptoms of respiratory muscle paralysis and autonomic neuropathy.
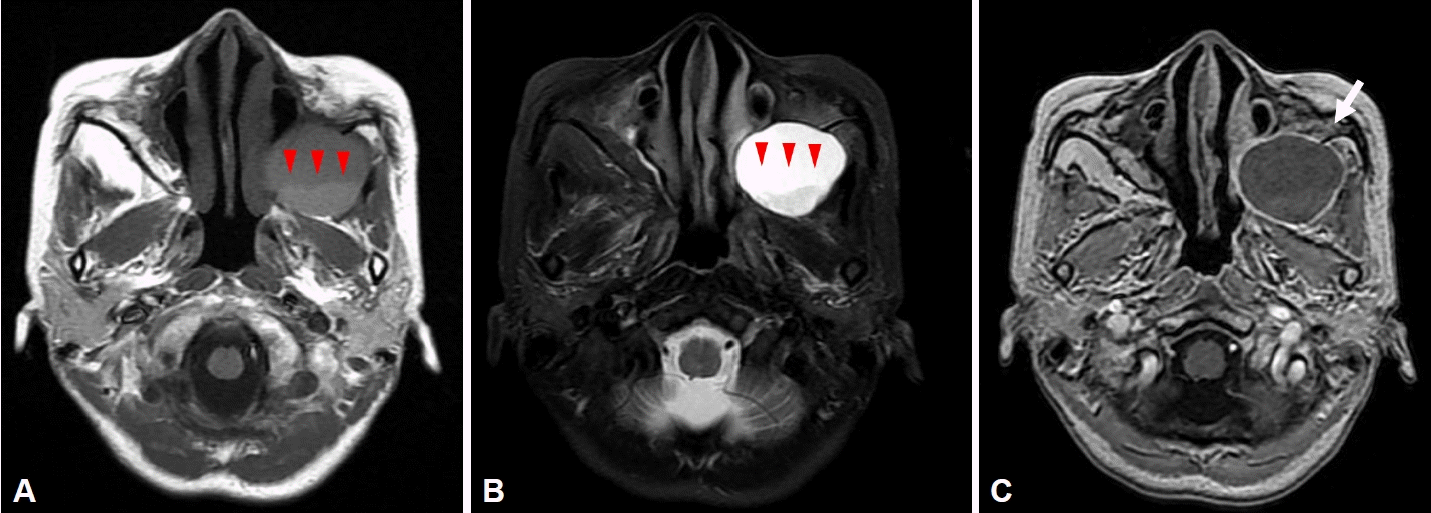 | Fig. 1.Preoperative magnetic resonance imaging findings. A: T1 axial view: a low-signal cystic lesion with a fluid-fluid level (red arrowheads) in the left maxillary sinus. B: T2 axial view: a high-signal cystic lesion with a fluid-fluid level (red arrowheads) in the left maxillary sinus. C: T1 gadolinium-enhanced axial view: a cystic lesion with rim enhancement (white arrow). 
|
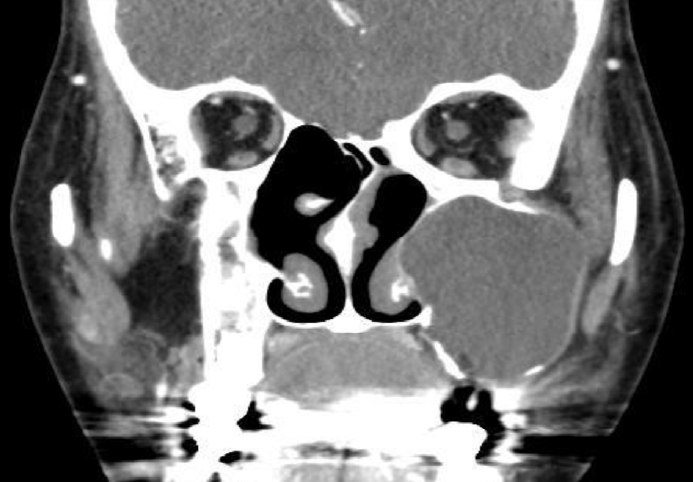 | Fig. 2.Preoperative computed tomography: a 4.2×3.8 cm hypodense homogenous cystic mass in the left maxillary sinus with bone thinning of the left maxillary sinus wall. 
|
Immunoglobulin was injected at a dose of 0.4 g/kg/day for 5 days. No new neurological symptoms, such as sensory symptoms, cranial nerve disorders, or autonomic nervous system abnormalities occurred during treatment in the intensive care unit. There was also no paralysis of the respiratory muscles. A nerve conduction velocity study showed acute axonal polyneuropathy in the lower extremities, which indicated acute motor axonal neuropathy (AMAN)-type GBS. The acute-phase treatment was completed, with partial improvements in the muscle strength of both lower extremities after intravenous immunoglobulin injections.
On the seventh day of hospitalization, the patient was transferred to a hospital near her hometown and outpatient rehabilitation treatment was conducted. Three follow-up observations were performed in the outpatient clinic thereafter. Ankle dorsiflexion muscle strength improved within 4 weeks, and the patient’s symptoms did not deteriorate within 8 weeks. The upper and lower extremity muscle strength was ankle dorsiflexion grade III and wrist flexion grade IV at the last outpatient visit.
Go to :

DISCUSSION
GBS causes rapidly progressing symmetrical paralysis and deterioration or loss of deep tendon reflexes, which can be accompanied by sensory symptoms or cerebral nerve paralysis. In the early 1900s, it was also called acute inflammatory demyelinating polyneuropathy (AIDP) based on the findings of demyelinating neuropathy on electrophysiological examinations. However, many studies have been conducted since then, and various types of GBS have been identified, including AIDP, AMAN, acute motor and sensory axonal neuropathy (AMSAN), and Miller Fisher syndrome have been identified. Each type shows different clinical symptoms and different responses to treatment.
Muscle weakness that moves up rapidly from the lower extremity to the upper extremity is a common symptom of GBS, but symptoms may begin in the proximal part or occur only in the lower extremity; with these presentations, GBS may be mistaken for spinal cord disease [
4]. Sensory symptoms appear as numbness or paresthesia. Pain manifests as back pain, muscle pain, and meningism [
1]. In 50% of patients, GBS is accompanied by cranial nerve disorders such as facial nerve palsy, extraocular motor dysfunction, and bulbar palsy. The National Institute of Neurological Disorders and Stroke diagnostic criteria, revised in 1990 by Asbury and Cornblath, required progressive paraplegia and loss of deep tendon reflexes, with other neurological symptoms, CSF findings, and nerve conduction tests supporting the diagnosis. In a CSF examination, albumin cell dissociation, in which the protein level in CSF is elevated although the number of cells does not increase, is known to be an important finding in diagnosing GBS. However, since albumin cell dissociation is observed more than 3 days after quadriplegia in 50% of patients, it may not be helpful for the early differential diagnosis [
5]. Protein elevation in the CSF is relatively common in AIDP, but tends to be not noticeable in AMAN or other subtypes of GBS; thus, repeated CSF tests for a diagnosis are not recommended [
6,
7]. In this case, Achilles tendon reflex loss appeared along with typical ascending quadriplegia, while sensory symptoms and cranial nerve palsy did not appear. On this basis, GBS could be diagnosed, and a nerve conduction study indicated that it was the AMAN type.
Approximately 60%–70% of GBS patients report showing symptoms of an infection, such as an upper airway or gastrointestinal infection, within 4 weeks of the neurological symptoms appearing [
6]. Representative causative microbes include
Campylobacter jejuni, Cytomegalovirus, Epstein-Barr virus, and
Mycoplasma pneumoniae. In particular, the outer cell membrane of
C. jejuni has a form of lipo-oligosaccharide similar to ganglioside GM1 or GD1a. According to molecular mimicry theory, the anti-ganglioside antibody formed by
Campylobacter infection produces a myelin-destructing complement, which finally induces the AMAN, AMSAN, or MFS type of GBS [
8].
Postoperative GBS is a complication in 4.1 patients per 100,000 operations, and the incidence of GBS within 6 weeks after surgery is 13.1 times higher than in individuals who do not undergo surgery [
9]. Symptoms appear within 1 week after surgery and peak within 4 weeks [
1]. Postoperative GBS most often occurs after gastrointestinal, cardiac, thoracic, neurological, or orthopedic surgery, as well as surgery in the female reproductive tract [
3]. In addition to age, infectious diseases, preexisting autoimmune diseases, and malignant diseases are associated with postoperative GBS [
3]. Of these factors, autoimmune disease and malignant disease are thought to have a particularly strong correlation with postoperative GBS. In this case, the patient had neither an autoimmune disorder nor malignancy. Nonetheless, her gastrointestinal infection history might have contributed to postoperative GBS.
Early studies of postoperative polyneuropathy postulated that it is caused by autoimmune action against nerve antigens released during surgery [
10]. Since then, it has been established that postoperative neuropathy can occur only through an inflammatory reaction without physical nerve damage, and histological examinations of nerves have confirmed an epineural perivascular lymphocytic inflammatory response and axonal degeneration [
11]. It is also known that the stress of surgery causes excessive secretion of adrenocorticotropic hormone and glucocorticoids, and activation of the sympathetic nervous system reduces the function of immune cells [
12]. It has also been hypothesized that lipid-soluble pharmacologic agents (e.g., halothane, enflurane, propofol, and ketamine) cause transient immunosuppression to nerve roots damaged by surgery, leading to a demyelinating disorder [
2]. In this case, it is thought that both surgery and infection contributed to the occurrence of GBS.
Intravenous immunoglobulin and plasma exchange have been proven to be effective treatments. Intravenous immunoglobulin at a dose of 0.4 g/kg day for 5 consecutive days is preferable to plasma exchange due to its availability, convenience, and minor complications [
1]. Admission to the Intensive care unit should be considered to monitor respiration and autonomic nerve dysfunction. Care should be taken when using steroids because studies have shown that they inhibit the progressive improvement of GBS. Aggravation may occur after symptom alleviation in 10% of patients in whom intravenous immunoglobulin is initiated, which is called treatment-related fluctuation (TRF). TRF occurs within 8 weeks, and the repeated administration of intravenous immunoglobulin is known to help treat TRF [
13]. Furthermore, if symptoms worsen more than three times or if symptoms worsen 8 weeks after symptoms occur, acute-onset chronic inflammatory demyelinating polyneuropathy should be included in the differential diagnosis [
14].
GBS generally improves substantially within a year, but patients slowly recover over a period of more than 3 years. If lower extremity weakness occurs after surgery, it is necessary to check whether this symptom is accompanied by respiratory muscle weakness, sensory symptoms, autonomic nerve dysfunction, and cranial nerve palsy. CSF tests and nerve conduction velocity studies help diagnose GBS. Even after appropriate treatment, regular follow-up is required for an accurate diagnosis and treatment. Rehabilitation treatment and mental support can be combined.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download