Abstract
Multiple primary malignancies (MPMs) are defined as the presence of two or more malignancies in different organs, without a subordinate relationship. Although rarely reported, hepatocellular carcinoma (HCC) occasionally presents with simultaneous or metachronous primary malignancies in other organs. In this report, we describe a patient with lung adenocarcinoma and lymph node and bone metastases, treated with five chemotherapeutic regimens for 24 months. Changing the chemotherapy regimen based on the suspicion of metastasis of a new liver mass did not lead to improvements. This prompted a liver biopsy and a revised diagnosis of HCC. Sixth-line treatment with the concurrent use of cisplatin-paclitaxel for lung cancer and sorafenib for HCC, stabilized the disease. The concurrent treatment was not tolerated and was discontinued owing to adverse events. Considering our findings, treatment with increased efficacy and lower toxicity for MPMs is warranted.
Multiple primary cancers refer to two or more malignancies occurring concurrently in the same or different organs, with unknown etiologies.1 Accordingly, hepatocellular carcinoma (HCC) occasionally presents with simultaneous or metachronous primary malignancies of other organs. Although HCC has only a low risk of multiple primary cancers compared to other primary cancers, its early diagnosis and comprehensive treatment improves survival in patients with cancer. Hence, the number of patients with HCC and multiple primary malignancies (MPM) has increased.2 Lung, colorectal, and thyroid cancers are common complications accompanying HCC.3 Chemotherapy has been used in simultaneously developing multiple unresectable cancers. In contrast, immune checkpoint inhibitors (ICIs) have been used to treat various malignancies based on their actions on the immune system, rather than directly acting on the cancer cells.4 Herein, we present a case of double primary cancers, featuring HCC with lung cancer. The Institutional Review Board of participating center (CR317022) waived the requirement for informed consent. This case report is described according to CARE guidelines (available at https://www.care-statement.org/).
A 69-year-old man with hypertension and diabetes mellitus was diagnosed with stage IIIC lung adenocarcinoma (cT3cN3cM1), with lymph node and bone metastases, in June 2019. The patient had no history of liver disease and had not consumed alcohol. Molecular testing revealed an L858R point mutation and a primary T790M mutation in the epidermal growth factor receptor exon 21 and exon 20, respectively. The tumor was weakly positive for programmed death-ligand 1 (PD-L1), with a tumor proportion score (TPS) of 10%, as determined by immunohistochemistry.
First-line treatment with gefitinib was initiated for 8 weeks and stopped due to hepatic dysfunction. Subsequently, afatinib was initiated as second-line treatment, and according to the response evaluation criteria in solid tumors 1.1 criteria, a partial response was observed (Fig. 1) for 14 months. The disease then progressed, with new liver involvement identified via chest computed tomography (CT). Pemetrexed was started. After two cycles, tumor progression was noted (Fig. 2A). Subsequently, fourth-line treatment with atezolizumab was initiated and continued for two cycles. However, this treatment could not be continued as its cost was not covered by National Health Insurance. Therefore, a fifth-line treatment, gemcitabine and vinorelbine, was initiated and used for 8 months. A CT scan revealed disease progression and the hepatic mass had increased in size, although the lung lesion was stably maintained. At this time, the treatment for lung cancer was changed to cisplatin-paclitaxel as a sixth-line treatment. However, there was no improvement in the liver mass, and the patient was referred to a hepatologist, who suggested a liver biopsy and revised the diagnosis for HCC.
At the time of HCC diagnosis, dynamic liver CT revealed a heterogeneous 18 cm HCC tumor, with portal and hepatic vein invasions in the right lobe (Fig. 2B). Complete blood count results were as follows: white blood cell count, 6.22×103/µL; hemoglobin, 9.8 g/dL; and platelet count, 369×103/µL. A liver function test demonstrated the following: serum aspartate aminotransferase, 113 U/L; alanine aminotransferase, 60 U/L; alkaline phosphatase, 160 U/L; total bilirubin 0.7 mg/dL, albumin 4.1 g/dL, and prothrombin time international normalized ratio, 1.16. Furthermore, the serum alphafetoprotein (AFP) was 278.6 ng/mL, whereas that of the protein induced by the absence of vitamin K or antagonist-II was 217,105 mAU/mL. Immunohistochemical analysis of the biopsy specimens confirmed pathologically well-differentiated HCC with a TPS of <10% of PD-L1 (Fig. 3). Mild hemochromatosis with pericellular fibrosis was also observed in the liver.
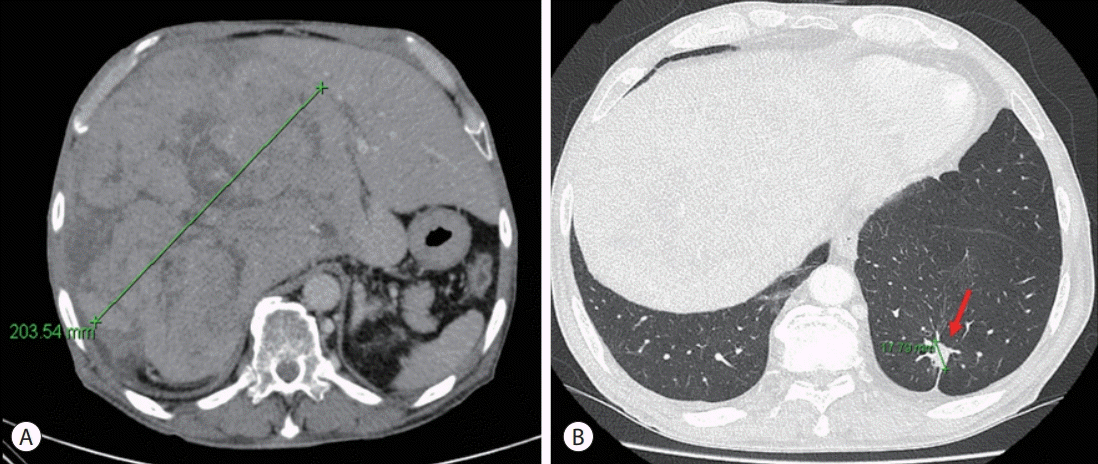
The patient was diagnosed with advanced HCC histologically and via image staging (modified Union for International Cancer Control stage IV, T4N0M0, and Barcelona Clinic Liver Cancer stage C). At this time, the patient’s Eastern Cooperative Oncology Group performance status was 0–1 and liver function was preserved. Hence, we first considered treatment with atezolizumab and bevacizumab for both lung cancer and HCC. However, as the drug combination was not covered by National Health Insurance, treatment for each cancer was considered. Subsequently, we included 400 mg sorafenib twice daily as the treatment for HCC and maintained cisplatin-paclitaxel as a sixth-line treatment for lung cancer. During treatment, liver function remained stable. After 2 months, when the treatment response was evaluated, we noted a slight size increase in the HCC mass. Liver CT revealed a maximum diameter of 18–20 cm (Fig. 4A). Tumor markers reflected positive therapeutic effects including a reduction of the serum AFP level from 278.6 to 273.3 ng/mL. Additionally, lung cancer assessment revealed a slight decrease in the diameter of the evaluable tumoral lesion from 2.1 to 1.8 cm (Fig. 4B). However, the combination treatment could not be continued because of adverse events including neutropenic fever and anorexia.
In this report, we described a patient whose newly developed HCC was misdiagnosed as lung metastases during his treatment for lung adenocarcinoma. It was challenging to decide on an appropriate treatment option because various systemic chemotherapies had already been used for lung adenocarcinoma treatment over a long time, and both primary cancers had to be collectively treated. Reportedly, approximately 40% of patients with MPM have a history of anticancer treatment or radiation therapy, and they consequently developed secondary tumors following initial treatment.5 Our patient was administered six chemotherapy regimens for lung cancer, which limited the treatment options for liver cancer. Moreover, long-term treatment could not be considered because of the toxicity from combination chemotherapy. Therefore, it is necessary to develop treatment options with greater efficacy and minimal treatment toxicity for the management of MPMs.
ICIs have been used to treat various malignancies by targeting the immune system, rather than directly affecting cancer cells, and have shown promising results in different cancers.6-9 ICIs can be strong first-line drug candidates for unresectable MPMs. Currently, inhibitors of cytotoxic T-lymphocyte-associated protein 4, programmed cell death protein 1 (PD-1), and its ligand (PD-L1), are well known and have been approved for several cancer treatments.10
Atezolizumab is a PD-L1 antibody that, along with bevacizumab, was recently approved for HCC.11 Additionally, it was approved in the USA and Europe as second-line treatment in advanced or metastatic non-small cell lung cancers (NSCLCs) in 2017.12 To date, the effect of atezolizumab on MPMs, including HCC and lung cancer, has not been reported. Only one report has shown the efficacy of atezolizumab in HCC and esophageal cancer.13 In that report, HCC treatment demonstrated a partial response and the esophageal cancer stabilized, and the patient remained healthy for 9 months with treatment.
Nivolumab is another monoclonal antibody targeting PD1, which is now the standard drug for various cancers including NSCLC, and has received accelerated approval for HCC in the US.14 Recently, the efficacy of nivolumab was reported in a patient with HCC and lung cancer.13 Persistent partial response of HCC and lung cancer was sustained for 9 months.
In conclusion, although MPMs associated with HCC are rare, the occurrence of HCC in patients with malignancies and the possibility of extrahepatic malignancies in patients with HCC should be considered. In terms of treatment, ICIs are effective in more than two types of cancer. ICIs are a promising treatment option for MPMs, including HCC. To verify this, further studies are warranted.
Notes
Ethics Statement
The Institutional Review Boards of all participating centers (CR317022) waived the requirement for informed consent.
References
2. Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007; 12:20–37.

3. Xu W, Liao W, Ge P, Ren J, Xu H, Yang H, et al. Multiple primary malignancies in patients with hepatocellular carcinoma: a largest series with 26-year follow-up. Medicine (Baltimore). 2016; 95:e3491.
4. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019; 7:306.

5. Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014; 6:119–134.

6. Dhandha M, Chu MB, Richart JM. Coexistent metastatic melanoma of the kidney with unknown primary and renal cell carcinoma. BMJ Case Rep. 2012; 2012:bcr2012007286.

7. Yamada H, Hida N, Satoh H, Yamagishi T, Hiroshima Y, Yoshii S, et al. Improved outcomes with pembrolizumab treatment in two cases of double cancer including non-small-cell lung cancer. Anticancer Drugs. 2019; 30:105–109.

8. Yamaguchi T, Sakurai K, Kuroda M, Imaizumi K, Hida T. Different response to nivolumab in a patient with synchronous double primary carcinomas of hypopharyngeal cancer and non-small-cell lung cancer. Case Rep Oncol. 2017; 10:802–808.

9. Nozawa Y, Oka Y, Oosugi J, Takemura S. Immunotherapy for pulmonary squamous cell carcinoma and colon carcinoma with pembrolizumab: a case report. Medicine (Baltimore). 2018; 97:e0718.
10. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018; 359:1350–1355.

11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.

12. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–265.

Figure 1.
Chest computed tomography (CT) and bone scan before and after chemotherapy for lung cancer. (A, C) Images on initial admission and (B, D) after second-line treatment with afatinib. The primary lesion (arrow) decreased and disappeared after chemoradiotherapy.
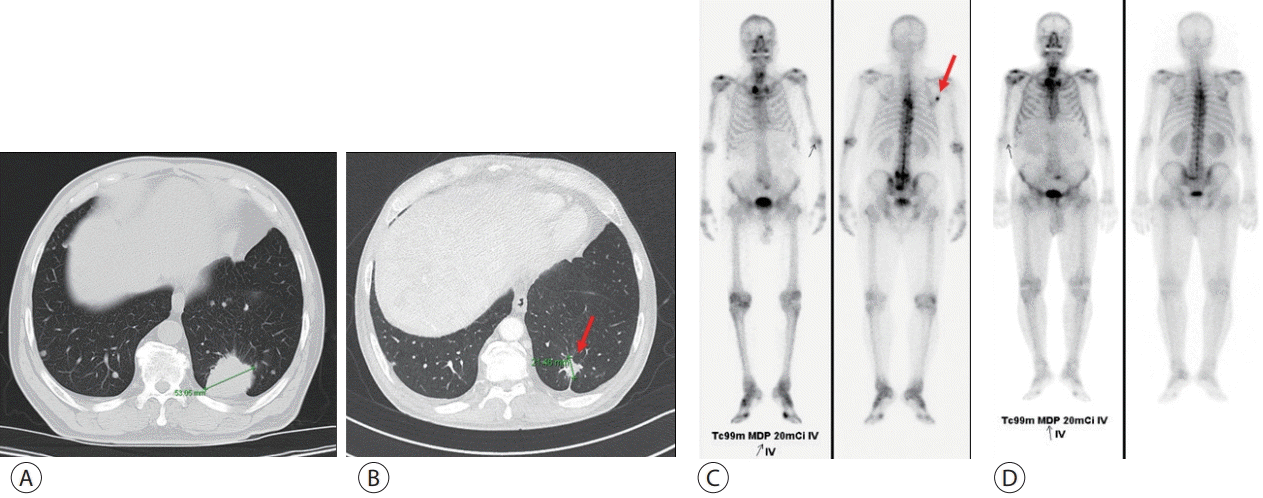
Figure 2.
(A) Follow-up chest CT showed a 14 cm tumor in the right lobe of the liver. (B) At the time of HCC diagnosis, liver dynamic CT revealed a heterogeneous 18 cm HCC with hepatic vein invasion. CT, computed tomography; HCC, hepatocellular carcinoma.
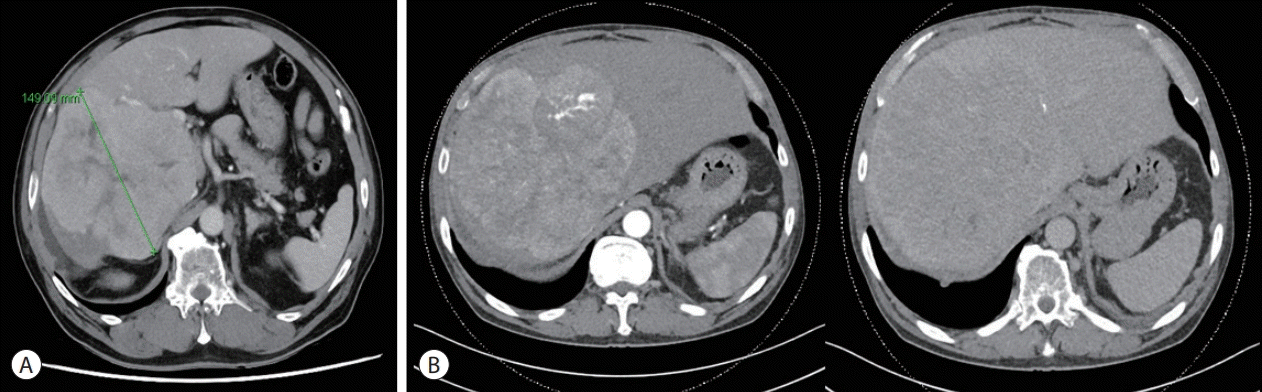
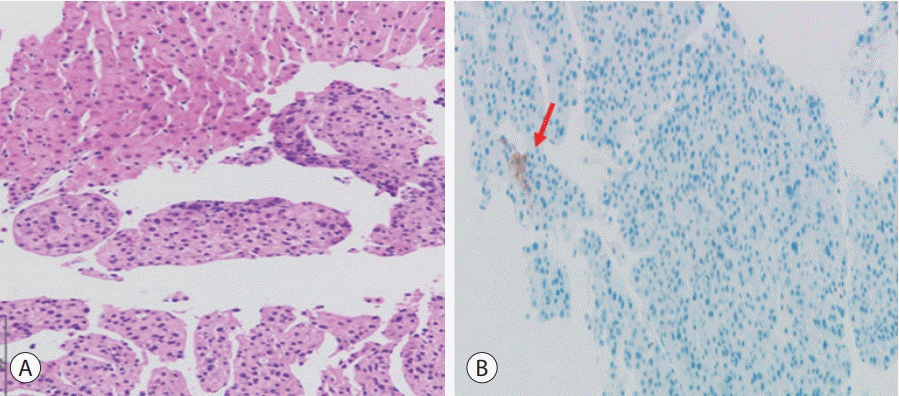
Figure 3.
Histopathological findings of the mass in the right hepatic lobe. (A) The biopsy specimens of the mass stained with hematoxylin and eosin demonstrate well-differentiated hepatocellular carcinoma. (B) On immunostaining, the tumor cells are weakly positive for membranous programmed death-ligand 1 expression (arrow).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download