This article has been
cited by other articles in ScienceCentral.
Abstract
Hepatoblastoma is an exceptionally rare malignancy in adults with just over 70 non-pediatric cases reported in literature. Recounted is a case of a 49-year-old female who presented with acute right upper quadrant abdominal pain, elevated serum alpha fetoprotein and a large liver mass on imaging. Hepatectomy was performed under clinical suspicion of hepatocellular carcinoma. Immunomorphologic characteristics of the tumor proved consistent with hepatoblastoma of mixed epithelial and mesenchymal type. Hepatocellular carcinoma remains to be the primary differential diagnosis for adult hepatoblastoma, however, distinguishing between these two neoplasms requires close histomorphologic assessment and immunohistochemical profiling as clinical, radiologic and gross pathologic findings typically overlap. Making this distinction is highly crucial in the timely initiation of surgical and chemotherapeutic interventions for this inherently aggressive and rapidly fatal disease.
Go to :

Keywords: hepatoblastoma, Carcinoma, hepatocellular, Pathology, surgical, Immunohistochemistry, Liver cancer
INTRODUCTION
Hepatoblastoma (HB) accounts for the vast majority of liver neoplasms in the pediatric population but is exceptionally rare in adults with 75 non-pediatric cases reported as of this writing.
1,
2 Hepatocellular carcinoma (HCC), the most common primary hepatic malignancy in adulthood, is understandably the foremost differential diagnosis for rare adult HB, however, distinguishing between these two disease entities may prove difficult to the encountering pathologist. Given the non-specific clinical, radiologic and gross pathologic manifestations of this malignancy, the diagnosis of HB in adults greatly relies on the correlation of distinct morphologic features and an overall immunohistochemical profile that sets it apart from HCC.
Go to :

CASE REPORT
A 49-year-old female presented acutely with a 10-day history of persistent and progressive abdominal pain localized to the right upper quadrant with radiation to the back. No accompanying symptoms were reported. On physical examination, hepatomegaly was elicited, however, stigmata of liver cirrhosis and signs of chronic liver disease were not observed. Whole abdominal ultrasound revealed the presence of an echogenic liver mass in the right hepatic lobe measuring approximately 12×10 cm on imaging. Liver function tests showed marked elevations in serum alanine transaminase (385 U/L), total bilirubin (12.3 mg/dL), direct bilirubin (8.7 mg/dL), and indirect bilirubin (3.6 mg/dL). Qualitative serological tests confirmed active infection with hepatitis B with reactivity for hepatitis B surface antigen and hepatitis B core antibody. Serum creatinine (2.1 mg/dL), lactate dehydrogenase (1,282 U/L) and C-reactive protein (30.94 mg/L) likewise reflected significantly increased values. Serum alpha fetoprotein (AFP) (>50,000 ng/mL) exceeded the assay limit of detection despite a 1:50 dilution, raising further suspicion for malignancy. The patient was subsequently admitted for surgery with a clinical impression of hepatocellular carcinoma in a background of cirrhosis secondary to hepatitis B infection for which the anti-viral Tenofovir was initiated.
A partial hepatectomy of the right liver lobe was performed. Serial sections of the 15.5×14×9.5 cm resection specimen showed a well-circumscribed, firm to friable, irregularly-shaped, grossly necrotic, white-tan mass measuring 12.5×11.8×9.8 cm with a distance of 1.5 cm from the nearest parenchymal resection margin. The rest of the liver surface appeared tan-yellow and non-cirrhotic.
Microscopic assessment of hematoxylin and eosin-stained sections revealed hepatic tissues infiltrated by a tumor with malignant epithelial and mesenchymal components. The epithelial component exhibited fetal, embryonal, cholangioblastic and macrotrabecular patterns with neoplastic cells having enlarged, hyperchromatic, ovoid to moderately pleomorphic nuclei, prominent nucleoli, coarse chromatin distribution, scanty eosinophilic cytoplasm and indistinct cell borders. The mesenchymal component displayed spindled cells in fascicular to haphazard arrangement with occasional bizarre, markedly pleomorphic cells identified. Brisk mitotic activity with frequent atypical mitotic figures were seen in both components. Osseous and chondroid metaplastic elements were noted. Extramedullary hematopoiesis and tumor invasion into lymphovascular and perineural structures were not observed (
Fig. 1).
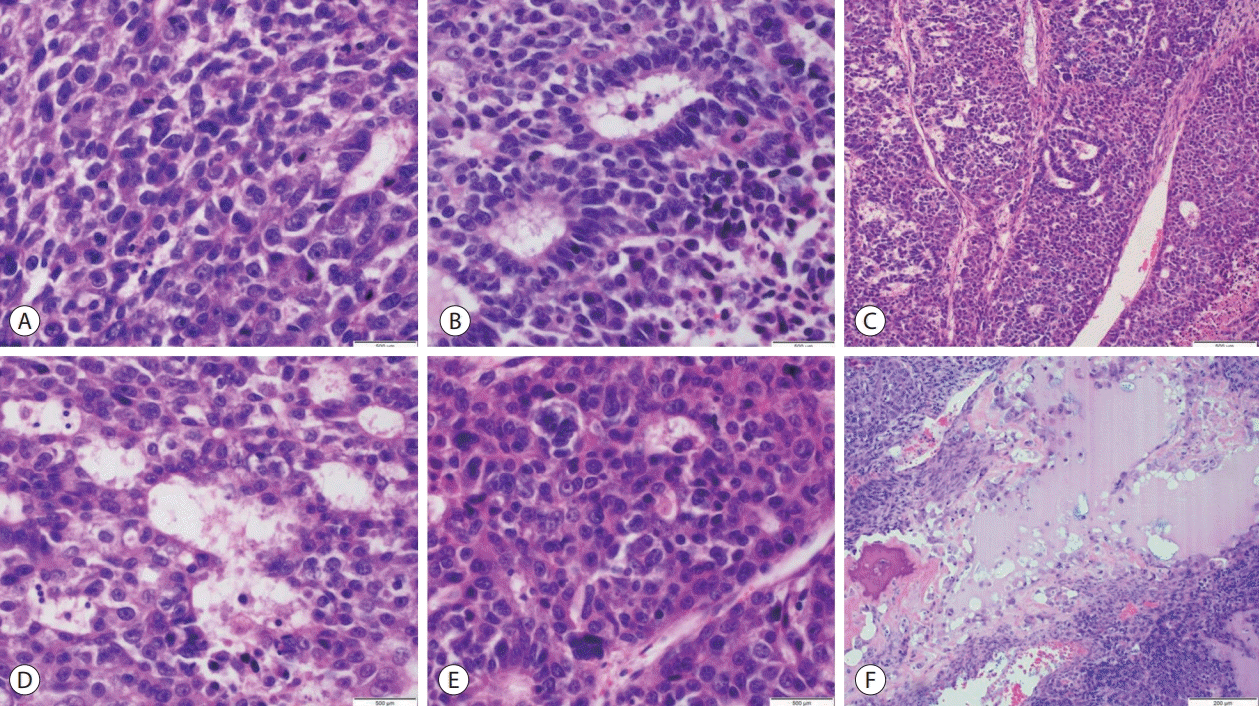 | Figure 1.Adult hepatoblastoma, mixed epithelial and mesenchymal type (A) fetal, crowded (mitotically active) pattern (hematoxylin and eosin [H&E], ×600). (B) Embryonal pattern (H&E, ×600). (C) Macrotrabecular pattern (H&E, ×400). (D) Cholangioblastic pattern (H&E, ×600). (E) Fetal pleomorphic pattern (H&E, ×600). (F) Osteoid formation (H&E, ×400). 
|
A panel of immunohistochemical stains was performed to aid in further characterization of the patient’s hepatic malignancy amid several distinct morphologic considerations. Tumor cells demonstrated diffuse and strong cytoplasmic staining for glypican-3 (GPC3), patchy and strong cytoplasmic expression for hepatocyte paraffin 1 (HepPar1) and cytokeratin (CK) 19, and positive nuclear expression for β-catenin. Strong cytoplasmic staining for CK7 was focally observed. Neoplastic cells did not stain for high-molecular weight CKs (
Fig. 2).
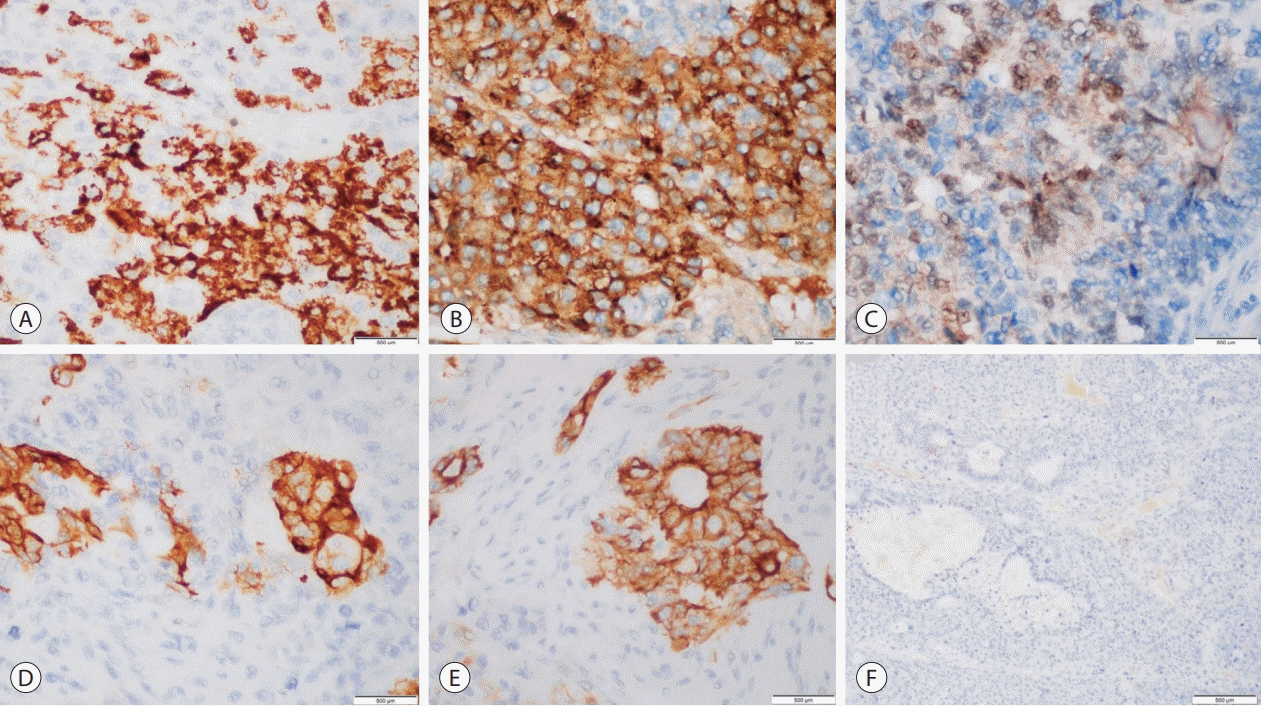 | Figure 2.Adult hepatoblastoma, immunohistochemistry. (A) Hepatocyte paraffin 1, cytoplasmic expression in fetal pattern (×600). (B) Glypican 3, coarse granular expression in fetal pattern (×600). (C) β-catenin, nuclear expression in fetal pattern (×600). (D) Cytokeratin (CK) 7, cytoplasmic expression in cholangioblastic pattern (×600). (E) CK19, cytoplasmic expression in cholangioblastic pattern (×600). (F) CK5/6, negative expression in neoplastic cells (×400). 
|
Following correlation of findings on histomorphologic assessment and immunohistochemical profiling, a diagnosis of adult hepatoblastoma, mixed epithelial and mesenchymal type was rendered. A systemic chemotherapy protocol consisting of cisplatin, vincristine and 5-fluorouracil was administered for four cycles, however, the patient succumbed to her malignancy less than 4 months from time of diagnosis.
Go to :

DISCUSSION
Hepatoblastoma is defined as malignant primary hepatic tumor that recapitulates hepatic organogenesis hence its predominantly primitive appearance with variable presence of epithelial and mesenchymal elements. Up to 90% of cases are known to occur in infancy to early childhood.
3 An excess of seventy cases have so far been reported in English literature since the entity was first described by Bartok in 1958.
1,
2,
4 Males and females are equally affected with a median age of 39 to 42 years in systematic reviews.
5,
6 Concrete risk factors for the occurrence of this malignancy in adults have yet to be identified, however, cases occurring in the setting of chronic viral hepatitis have been reported.
6 Right upper quadrant abdominal pain is the most commonly reported symptom at presentation which may be accompanied by abdominal enlargement, anorexia and weight loss. Jaundice is rarely observed. Elevation of serum AFP is seen in the vast majority of cases, however, low to normal levels may be associated with the particularly aggressive small cell undifferentiated subtype.
3,
6 The radiologic qualities of this tumoral entity are relatively non-specific.
2
Hepatoblastomas are typically well-delineated, solitary or multiple lesions, bordered by a thin pseudocapsule, with a nodular or bosselated cut surface that may appear tan-brown or variegated with areas of cystic degeneration, necrosis and hemorrhage.
3 The right lobe is more often affected and the background liver tissue is usually non-cirrhotic.
2,
6 Histologically, the International Pediatric Liver Tumor Consensus Classification outlines two major types, epithelial HB or mixed epithelial and mesenchymal HB. The epithelial component is morphologically variable and may consist of a combination of fetal, embryonal, small cell undifferentiated, cholangioblastic and macrotrabecular patterns. The mesenchymal components of mixed type HB, the most common histologic type observed in adults, may be composed of fibrous, osseous or cartilaginous elements.
3,
6,
7 It is notable that adult HB shares greater histologic similarities with HCC in contrast to pediatric cases.
1 This morphologic diversity in the setting of non-specific clinical and radiologic manifestations make the distinction between HCC and adult HB rather challenging. Rougemont et al.
8 delineated three main histologic criteria to distinguish HB from HCC, namely, presence of a “light and dark” pattern, extramedullary hematopoiesis and mesenchymal elements.
Although there exists not one particular marker to differentiate HB from HCC,
1,
6 a roster of immunohistochemical stains may help evince the immature nature of this malignancy. In particular, epithelial and mesenchymal fetal components display nuclear and cytoplasmic expression for betacatenin and overexpress glutamine synthetase as a result of activation of the
Wnt signaling pathway. GPC3 is more likely expressed in embryonal and fetal epithelial components manifesting as fine to coarse granular cytoplasmic staining. HepPar1, though expressed in the fetal component, remains negative in more primitive embryonal areas. Cholangioblastic elements stain for CK7 and CK19 but typically lack expression against markers for GPC3, glutamine synthetase (GS) and HepPar1. Switch/sucrose non fermentable (SWI/ SNF) related, matrix associated, actin dependent regulator of chromatin subfamily B member 1 (SMARCB1) or integrase interactor 1 (INI-1) nuclear expression is retained in all components except in the small cell undifferentiated subtype (
Table 1).
3,
7
Table 1.
Immunohistochemical profiles of adult hepatoblastoma and hepatocellular carcinoma
3,
7,
10-
12
|
Adult hepatoblastoma |
Hepatocellular carcinoma |
|
β-Catenin |
Nuclear expression in fetal, embryonal and SCUD patterns; some membranous expression in fetal areas |
Membranous expression in HCC (-80%); cytoplasmic or nuclear co-expression infrequently seen in poorlydifferentiated HCC; solitary nuclear reactivity does not occur |
|
GPC 3 |
Fine to coarse granular cytoplasmic expression in fetal and embryonal areas |
Granular cytoplasmic expression in poorly-differentiated HCC; negative in well-differentiated HCC and non-neoplastic liver |
|
HepPar1 |
Cytoplasmic expression in fetal areas; negative in embryonal, SCUD and cholangioblastic areas |
Cytoplasmic expression in well-differentiated HCC; loss of expression seen in poorly-differentiated HCC |
|
GS |
Cytoplasmic expression in fetal areas; variably expressed in embryonal areas; negative in SCUD and cholangiobastic areas |
Cytoplasmic expression in HCC; used in tandem with HSP70 to detect well-differentiated HCC |
|
CK 7 |
Cytoplasmic expression in cholangioblastic areas |
Negative expression |
|
CK 19 |
Cytoplasmic expression in cholangioblastic areas; variably expressed in SCUD pattern |
Expressed in biphenotypic HCC (10–30% of HCC), associated with aggressive behavior, tumor recurrence and poorer outcomes |

Genomic profiling of HBs reveals its complex pathogenesis with activation of the
Wnt signaling pathway detected in 90% of cases arising secondarily to somatic mutations in the
CTNNB1 gene and less commonly in other
Wnt signaling genes. There have also been efforts to molecularly subtype these tumors in aid of prognostication and risk stratification. High risk molecular features include high
HIF1A activity, high
NFE2L2, LIN28B, HMGA2, SALL4 and
AFP expression, and low
let-7 expression.
9
Surgery remains to be the mainstay of treatment despite lack of standardized approaches in the management of adult HB. Cisplatin-based systemic chemotherapy regimens are likewise recommended but therapeutic response is extremely poor as most cases are advanced at presentation.
1 These tumors are known to portend a negative prognosis, especially with increasing age, with a median survival of 2 months and survival rates as low as 16.7% within a year of diagnosis for patients aged 45 years and older.
6
Adult hepatoblastoma is an extremely rare primary hepatic malignancy with diverse morphology and non-specific immunohistochemical expression posing a significant diagnostic challenge amid histologic consideration of the more prevalent hepatocellular carcinoma. Taken together, the recognition of distinct epithelial patterns with respective appropriate reactivity against a panel of β-catenin, GS, GPC3, HepPar1, CK7 and CK19 may alert the pathologist to the presence of immature tumoral components simulating hepatic organogenesis. Knowledge of the possibility of admixed mesenchymal elements being more common in adult populations and lack of association with liver cirrhosis may also help clinch the diagnosis.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download