INTRODUCTION
CASE REPORT
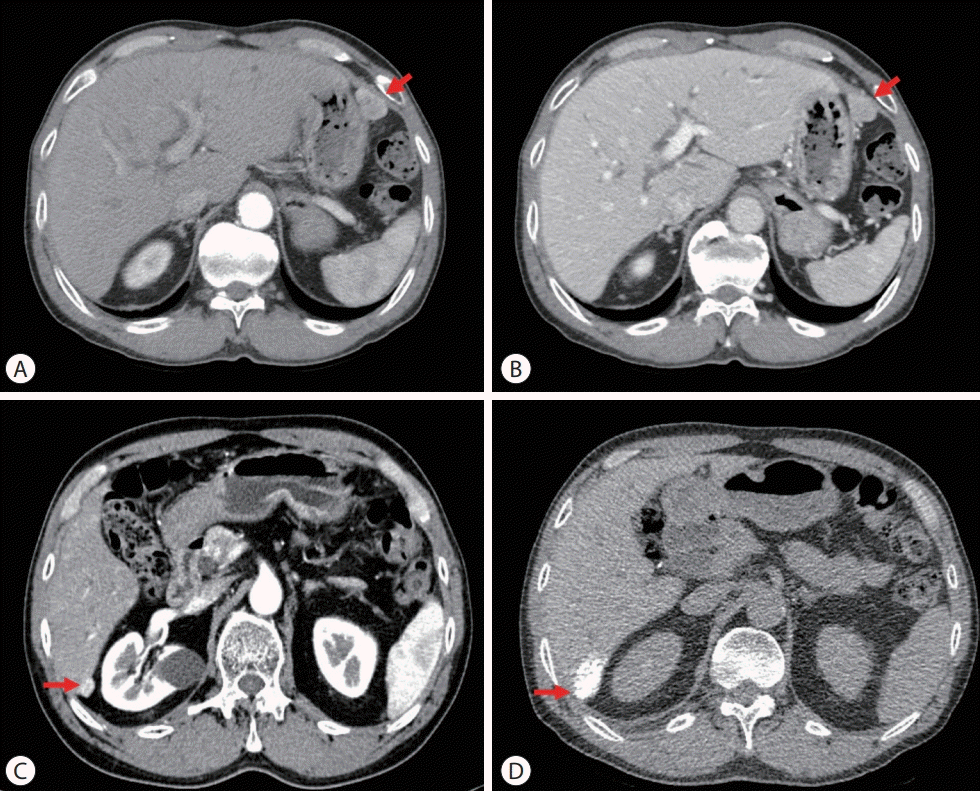 | Figure 1.Initial and follow-up liver dynamic computed tomography (CT) imaging findings. (A) Arterial and (B) portal phase on July 2012, 3.5 cm single nodular hepatocellular carcinoma (HCC) on S3 (red arrow). (C) Arterial phase, before transarterial chemoembolization (TACE) on May 2014, 1.5 cm recurred HCC on S6 (red arrow). (D) Lipiodol uptake (red arrow) on non-contrast CT after TACE on July 2014. |
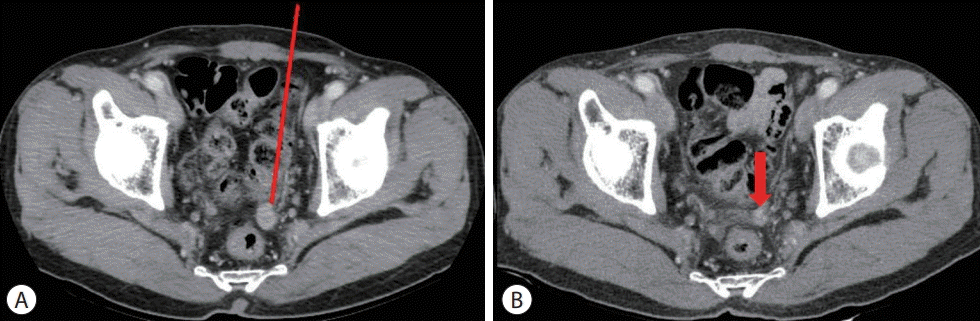 | Figure 2.Follow-up liver dynamic computed tomography (CT) imaging findings before and after radiotherapy transarterial chemoembolization. (A) Liver dynamic CT, arterial phase, before radiotherapy on October 2014, 1 cm nodule on the rectovesical pough (red arrow). (B) Liver dynamic CT, arterial phase, 5 mm nodule (red arrow) after radiotherapy on June 2015. |
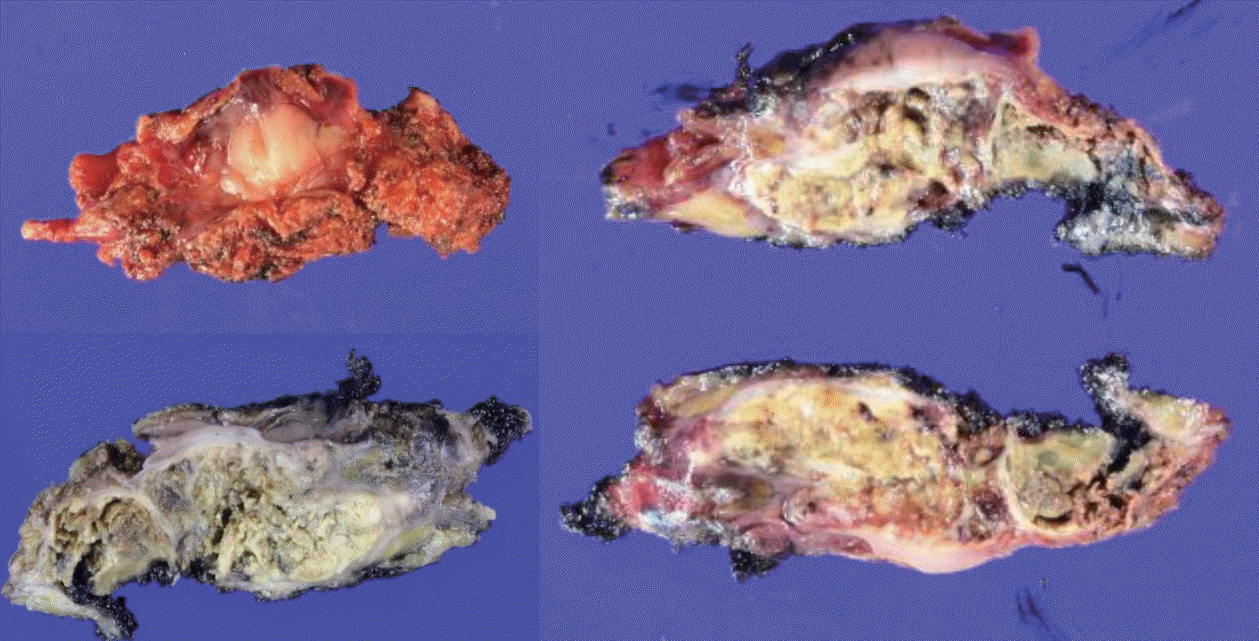
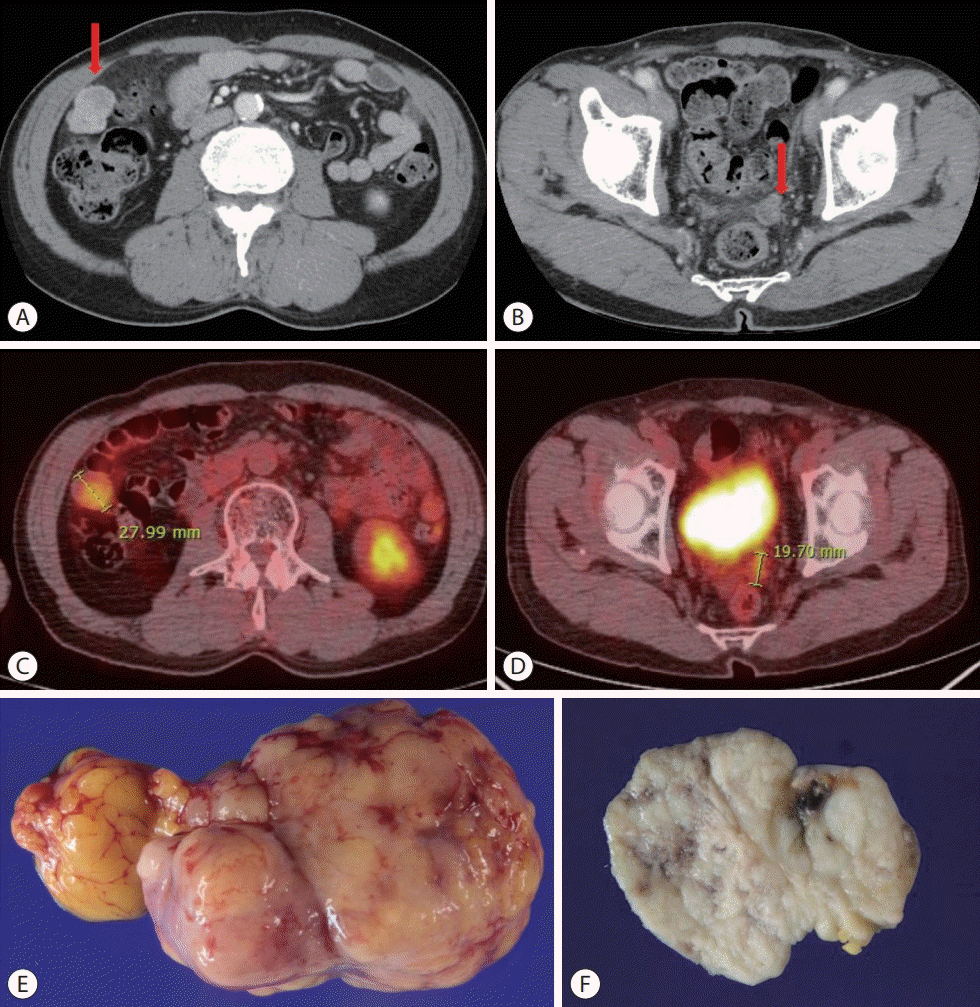 | Figure 3.Follow-up liver dynamic computed tomography (CT) and positron emission tomography (PET)/CT imaging findings on May 2017: the progression of peritoneal metastases and omental metastatic mass of hepatocellular carcinoma after surgical resection on June 2017. (A) Liver dynamic CT, arterial phase, right upper quadrant area on abdominal cavity (red arrow). (B) Liver dynamic CT, arterial phase, rectovesical pouch (red arrow). (C) PET/CT, 2.8 cm sized nodule on right upper quadrant area on abdominal cavity. (D) PET/CT, 1.97 cm sized nodule on the rectovesical pouch. (E) Mass on right upper quadrant area on abdominal cavity. (F) Mass on the rectovesical pouch. |
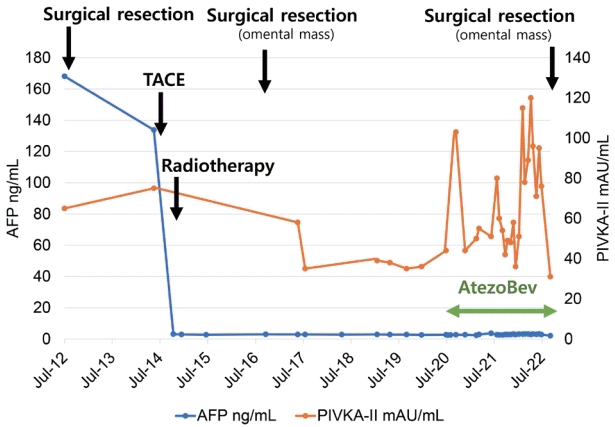
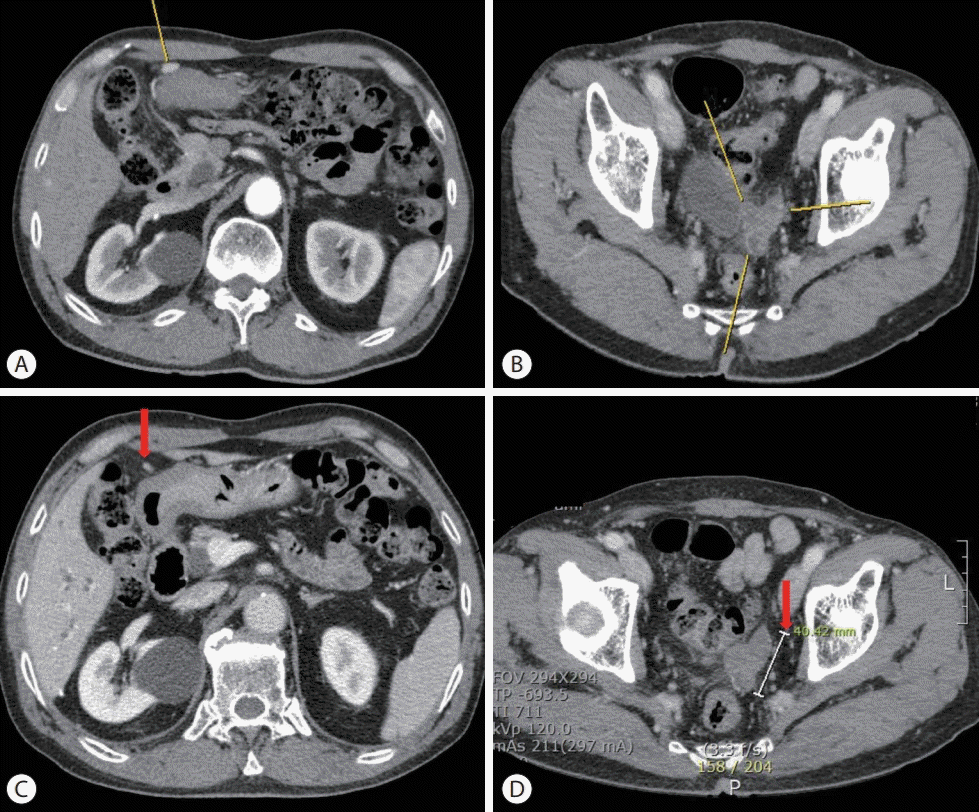 | Figure 4.Follow-up liver dynamic computed tomography (CT) imaging findings before and after systemic therapy: the recurrence of peritoneal metastases. (A) Liver dynamic CT, arterial phase, right upper quadrant area on abdominal cavity (yellow line) on June 2020. (B) Liver dynamic CT, arterial phase, rectovesical pouch (yellow line) on June 2020. (C) Liver dynamic CT, arterial phase, right upper quadrant area on abdominal cavity (red arrow) on June 2021 after 33 cycles of atezolizumab and bevacizumab combination therapy. (D) Liver dynamic CT, arterial phase, rectovesical pouch (red arrow) on June 2021 after 33 cycles of atezolizumab and bevacizumab combination therapy. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download