This article has been
cited by other articles in ScienceCentral.
Abstract
Background/Aim
This study aimed to determine the diagnostic performance of 2022 Korean Liver Cancer Association-National Cancer Center (KLCA-NCC) imaging criteria compared with the 2018 KLCA-NCC for hepatocellular carcinoma (HCC) in high-risk patients using magnetic resonance imaging (MRI).
Methods
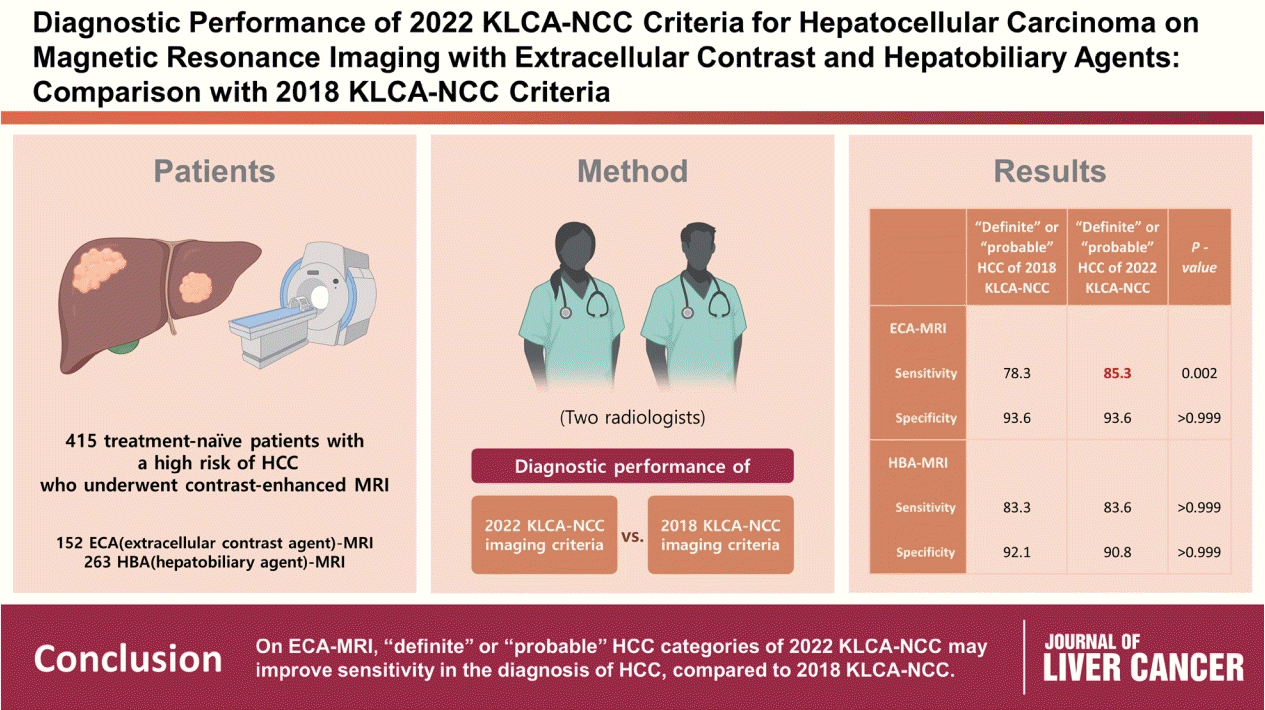
This retrospective study included 415 treatment-naïve patients (152 patients who underwent extracellular contrast agent [ECA]-MRI and 263 who underwent hepatobiliary agent [HBA]-MRI; 535 lesions, including 412 HCCs) with a high risk of HCC who underwent contrast-enhanced MRI. Two readers evaluated all lesions according to the 2018 and 2022 KLCA-NCC imaging diagnostic criteria, and the per-lesion diagnostic performances were compared.
Results
In “definite” HCC category of both 2018 and 2022 KLCA-NCC, HBA-MRI showed a significantly higher sensitivity for the diagnosis of HCC than ECA-MRI (77.0% vs. 64.3%, P=0.006) without a significant difference in specificity (94.7% vs. 95.7%, P=0.801). On ECAMRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC had significantly higher sensitivity than those of the 2018 KLCA-NCC (85.3% vs. 78.3%, P=0.002) with identical specificity (93.6%). On HBA-MRI, the sensitivity and specificity of “definite” or “probable” HCC categories of both 2018 and 2022 KLCA-NCC were not significantly different (83.3% vs. 83.6%, P>0.999 and 92.1% vs. 90.8%, P>0.999, respectively).
Conclusions
In “definite” HCC category of both 2018 and 2022 KLCA-NCC, HBA-MRI provides better sensitivity than ECA-MRI without compromising specificity. On ECA-MRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC may improve sensitivity in the diagnosis of HCC compared with the 2018 KLCA-NCC.
Keywords: Hepatocellular carcinoma, Diagnosis, Magnetic resonance imaging, Contrast media
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common type of primary hepatic malignancy.
1 Depending on the high pretest probability of HCC, it can be non-invasively diagnosed based on imaging features without mandatory pathologic confirmation in high-risk populations.
2 Thus, reliable guidelines for the non-invasive imaging diagnosis of HCC are crucial.
2,
3
After its initial announcement in 2003, the Korean Liver Cancer Study Group (KLCSG)-National Cancer Center (NCC) Korea practice guidelines for the management of HCC have been revised in 2009 and 2014.
4-
6 Since then, the Korean Liver Cancer Association (KLCA, formerly KLCSG)-NCC published the 2018 KLCA-NCC HCC practice guideline and recently updated it in 2022.
7-
11 They provide diagnosis, staging, and treatment guidelines for HCC specific to Asia, especially Korea.
7-
11 The 2018 and 2022 KLCA-NCC HCC practice guidelines assign a lesion into “indeterminate” nodule, “probable” HCC, or “definite” HCC categories after excluding benign lesions such as hemangiomas based on marked T2 hyperintensity and other malignancies such as cholangiocarcinoma based on targetoid appearance.
7-
11 According to the 2018 KLCA-NCC HCC practice guideline, non-invasive diagnosis of “definite” HCC is based on the typical imaging hallmarks of HCC defined as arterial phase hyperenhancement (APHE) with washout in the portal venous phase (PVP), delayed phase (DP), or hepatobiliary phase (HBP) on multiphase computed tomography or multiphase magnetic resonance imaging (MRI) with extracellular contrast agents (ECAs) or hepatobiliary agents (HBAs).
7,
8 The recently updated 2022 KLCA-NCC imaging criteria retained the expanded washout appearance on HBA-MRI without changes to “definite” HCC category.
9-
11 In contrast, the imaging criteria for “probable” HCC were modified in the 2022 KLCA-NCC to make their application depend on whether APHE is present.
9-
11
To the best of our knowledge, external validation of the recently updated 2022 KLCA-NCC imaging criteria has not yet been published, and there are no studies comparing the 2022 KLCA-NCC and the previous 2018 KLCA-NCC imaging criteria on ECA-MRI and HBA-MRI. Therefore, this study aimed to determine the diagnostic performance of the updated 2022 KLCA-NCC imaging criteria compared with the 2018 KLCA-NCC for the diagnosis of HCC in high-risk patients using ECA-MRI and HBA-MRI.
METHODS
Patients
We retrospectively searched our institution’s databases for a clinical cohort of HCC surveillance and identified 3,795 patients who underwent MRI for further diagnostic workup. The inclusion criteria were as follows: (1) age ≥18 years; (2) high risk for HCC with liver cirrhosis or chronic hepatitis B; (3) no previous treatment for hepatic lesions; and (4) presence of at least one and up to five hepatic lesions (each ≥1 cm) on MRI. We excluded patients with insufficient final diagnosis such as unknown final diagnosis of malignancy due to immediate locoregional therapy, or insufficient follow-up (<2 years) for benign lesions to determine size stability.
MRI examination
MRI was performed using a 3.0-T (Magnetom Trio Tim, Siemens Healthineers, Erlangen, Germany; Intera Achieva, Ingenia, or Ingenia CX, Philips Healthcare, Best, the Netherlands; and Discovery MR 750w, GE Healthcare, Waukesha, WI, USA) or 1.5-T system (Intera Achieva, Philips Medical Systems, Best, the Netherlands). The protocol included the acquisition of dual-echo T1-weighted gradient-echo images (in-phase and opposed-phase), T1-weighted three-dimensional gradient-echo images with dynamic contrast enhancement, navigator-triggered single- or multi-shot T2-weighted images, and diffusion-weighted images at b-values of 0 or 50, 400, and 800 s/mm2. Dynamic T1-weighted imaging was performed before and after administration of one of the three ECAs (gadoterate meglumine, Dotarem, Guerbet SA, Aulnay-sous-Bois, France; gadopentetate dimeglumine, Magnevist or gadobutrol, Gadovist, Bayer Pharma AG, Berlin, Germany) or HBA (gadoxetate disodium, Primovist, Bayer Pharma AG). The choice of MRI contrast agent (ECA or HBA) was based on the referring physicians’ discretion. They were fully informed by radiologists regarding the advantages and disadvantages of each contrast material at a multidisciplinary conference at our institution, such as potential differences in terms of the degree of arterial phase (AP) enhancement and relative frequency of artifacts between the two contrast materials, potential benefits of HBP images, and cost of contrast materials. AP scanning was initiated using the test-bolus or bolus-tracking technique, and PVP (60 seconds), 3 minutes DP, and 20 minutes HBP images (only after HBA administration) were evaluated.
Image analysis
Two board-certified abdominal radiologists retrospectively and independently reviewed all the images. They were blinded to the final diagnosis of each lesion but were aware that the study population consisted of patients at high risk for HCC. Both 2018 and 2022 KLCA-NCC criteria were applied to each lesion by the two readers. After independent categorization, the inter-reader agreement was evaluated. In cases of discrepancy between the two readers, the final category was determined by consensus.
2018 KLCA-NCC IMAGING CRITERIA
According to the 2018 KLCA-NCC criteria, the readers evaluated the presence or absence of major and ancillary imaging features and targetoid appearance.
7,
8 Each lesion was assigned as “definite” HCC, “probable” HCC, or “indeterminate” in a stepwise manner after excluding marked T2 hyperintensity or targetoid appearances.
7,
8 Targetoid appearance was assessed on diffusion-weighted imaging (DWI) or contrast-enhanced sequences.
7,
8 Non-invasive diagnosis of “definite” HCC is based on the typical imaging hallmarks of HCC. The major imaging features for a “definite” diagnosis of HCC were defined as APHE with washout in the PVP, DP, or HBP.
7,
8 In a lesion with some but not all of the major imaging features of HCC, “probable” HCC was assigned only when the lesion fulfilled at least one item from each of the following two categories of ancillary imaging features: favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, HBP hypointensity, and threshold growth) and favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, and fat or blood products in mass).
7,
8 When the imaging diagnosis was inconclusive, a lesion was defined as indeterminate.
7,
8
2022 KLCA-NCC imaging criteria
According to the 2022 KLCA-NCC criteria, the readers assessed the presence or absence of radiological hallmarks of HCC, ancillary imaging features, and targetoid appearances.
9-
11 Each lesion was categorized as “definite” HCC, “probable” HCC, or “indeterminate” nodule in a stepwise manner after excluding marked T2 hyperintensity or targetoid appearances, same as the 2018 KLCA-NCC.
9-
11 Targetoid appearances were evaluated on DWI or contrast-enhanced images.
9-
11 “Definite” HCC was defined as a lesion with APHE with washout appearance in the PVP, DP, or HBP, identical to the 2018 KLCA-NCC criteria.
9-
11 Lesion that did not fulfill the non-invasive diagnostic criteria of HCC was diagnosed as “probable” HCC if it met the following criteria: (1) APHE with at least one of the ancillary imaging features suggesting malignancy in general (group A: mild-to-moderate T2 hyperintensity, high signal intensity on DWI, and threshold growth) or favoring HCC in particular (group B: enhancing or non-enhancing capsule, mosaic architecture, nodule-innodule appearance, and fat or blood products in mass), or (2) no APHE with at least one ancillary imaging features from each group.
9-
11 When imaging features did not fulfill the abovementioned criteria, the lesion was defined as “indeterminate” nodule.
9-
11
Reference standards
The diagnoses of HCCs and non-HCC malignancies were confirmed by pathology, including surgical resection (n=427) and explants for transplantation (n=28). Benign diagnoses were obtained by pathology (n=15) or typical imaging features and stability at imaging for at least 2 years (n=65).
Statistical analysis
The baseline characteristics of patients and lesions were compared between the ECA-MRI and HBA-MRI groups using Fisher’s exact test for categorical variables and two-sample t-test for continuous variables. The per-lesion sensitivities and specificities of the 2018 and 2022 KLCA-NCC imaging criteria were calculated (
Supplementary Table 1) and compared using McNemar’s test. The chi-square test was conducted to compare the diagnostic performances of the 2018 and 2022 KLCA-NCC criteria between the two independent ECA-MRI and HBA-MRI groups. Inter-reader agreements for the categorization of lesions according to the 2018 and 2022 KLCA-NCC criteria were evaluated using Cohen’s κ coefficient. The κ values were interpreted as follows: poor, 0.00–0.20; fair, 0.21–0.40; moderate, 0.41–0.60; good, 0.61–0.80; and excellent, 0.81–1.00. Statistical analyses were performed using MedCalc version 16.2.1 (MedCalc Software, Ostend, Belgium). A
P-value <0.05 was considered statistically significant.
RESULTS
Characteristics of patients and lesions
The characteristics of the patients and lesions are presented in
Table 1. A total of 415 patients (mean age, 57.3 years; 292 men and 123 women) with 535 lesions were included. This study included 152 patients with 190 lesions who underwent ECA-MRI and 263 patients with 345 lesions who underwent HBA-MRI. There were no significant differences in sex, age, etiology of liver disease, presence of liver cirrhosis, lesion size, and final diagnosis between the ECA-MRI and HBA-MRI groups. Hepatitis B (87.7%) was the predominant etiology of the underlying liver disease. The 535 lesions included 412 (77.0%) HCCs, 43 (8.0%) non-HCC malignancies, and 80 (15.0%) benign lesions.
Diagnostic performances of “definite” HCC category according to the 2018 and 2022 KLCA-NCC criteria
On ECA-MRI, the sensitivity and specificity of the “definite” HCC category were 64.3% (95% confidence interval [CI], 55.9–72.2%) and 95.7% (95% CI, 85.5–99.5%), respectively, for diagnosing HCC according to both criteria. On HBA-MRI, the sensitivity and specificity of “definite” HCC category were 77.0% (95% CI, 71.5–81.9%) and 94.7% (95% CI, 87.1–98.6%), respectively. Sensitivities and specificities of “definite” HCC category were identical for both 2018 and 2022 KLCA-NCC criteria.
Diagnostic performances of “definite” or “probable” HCC categories according to the 2018 and 2022 KLCA-NCC criteria
Table 2 and
Supplementary Table 2 demonstrates the diagnostic performances for “definite” or “probable” HCC categories of the 2018 and 2022 KLCA-NCC criteria on ECA-MRI and HBA-MRI.
On ECA-MRI, the sensitivity was significantly higher for “definite” or “probable” HCC categories of the 2022 KLCA-NCC than those of the 2018 KLCA-NCC (85.3% [95% CI, 78.4–90.7%] vs. 78.3% [95% CI, 70.7–87.8%], P=0.002) without a difference in specificity (both, 93.6% [95% CI, 82.5–98.7%], P>0.999). On HBA-MRI, the sensitivity and specificity of “definite” or “probable” HCC categories of the 2018 and 2022 KLCA-NCC criteria were not significantly different (83.3% [95% CI, 78.3–87.5%] vs. 83.6% [95% CI, 78.7–87.9%], P>0.999; and 92.1% [95% CI, 83.6–97.1%] vs. 90.8% [95% CI, 81.9–96.2%], P>0.999, respectively).
Comparison of diagnostic performances for ECA‑MRI and HBA‑MRI according to the 2018 and 2022 KLCA-NCC criteria
Table 3 and
Supplementary Table 3 shows a comparison of the diagnostic performances of ECA-MRI and HBA-MRI according to the 2018 and 2022 KLCA-NCC criteria.
In “definite” HCC category of both 2018 and 2022 KLCA-NCC criteria, HBA-MRI showed a significantly higher sensitivity than ECA-MRI (77.0% [95% CI, 71.5–81.9%] vs. 64.3% [95% CI, 55.9–72.2%], P=0.006), whereas the specificity was not significantly different (94.7% [95% CI, 87.1–98.6%] vs. 95.7% [95% CI, 85.5–99.5%], P=0.801). In “definite” or “probable” HCC categories of both 2018 and 2022 KLCA-NCC criteria, no statistically significant differences in sensitivity and specificity were observed between ECA-MRI and HBA-MRI (all, P≥0.218).
Inter-reader agreement
The inter-reader agreement for the categorization of lesions according to the 2018 and 2022 KLCA-NCC criteria was excellent (κ=0.88 [95% CI, 0.84–0.91] and 0.89 [95% CI, 0.85–0.93], respectively).
DISCUSSION
The present study demonstrated that HBA-MRI showed a higher sensitivity for “definite” HCC category than ECA-MRI without a significant difference in specificity according to the 2018 and 2022 KLCA-NCC criteria. On ECA-MRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC criteria provided better sensitivity than those of the 2018 KLCA-NCC with identical specificity.
The 2018 KLCA-NCC imaging criteria defined washout in the PVP, DP, or HBP, and the recently revised 2022 KLCA-NCC criteria maintained an extended definition of washout appearance on HBA-MRI.
7-
11 In both 2018 and 2022 KLCA-NCC imaging criteria, “definite” diagnosis of HCC was the same and was defined as APHE with a washout appearance.
7-
11 When hypointensity in the HBP is considered as washout appearance, it is known that sensitivity is increased.
12-
14 Given the Korean medical environments where HBA-MRI is widely used, and early HCC detection and treatment are emphasized, the diagnostic criteria for “definite” HCC of the 2018 and 2022 KLCA-NCC were designed to favor high sensitivity on HBA-MRI.
7,
8,
15 The present study verified that HBA-MRI provides higher sensitivity than ECA-MRI in “definite” HCC category. Nonetheless, the specificity of HBA-MRI was not significantly impaired in “definite” HCC category compared with that of ECA-MRI. The imaging criteria for the diagnosis of HCC according to the 2018 and 2022 KLCA-NCC may have high specificity for both ECA-MRI and HBA-MRI by excluding benign lesions such as hemangiomas based on marked T2 hyperintensity and other malignancies such as cholangiocarcinoma based on targetoid appearance on DWI or contrast-enhanced sequences.
7-
11
Unlike “definite” HCC category, “probable” HCC category was changed in the updated 2022 KLCA-NCC.
7-
10 In the 2018 KLCA-NCC imaging criteria, “probable” HCC can be assigned only when a lesion fulfills at least one item from each of the following two groups of ancillary imaging features; favoring malignancy in general and favoring HCC in particular, regardless of the presence or absence of APHE.
7,
8 In the updated 2022 KLCA-NCC, nodules without APHE can be assigned as “probable” HCC only if the lesion meets at least one ancillary imaging features from each group. In contrast, nodule with APHE but no washout appearance can be more easily classified into “probable” HCC category when a lesion fulfills at least one of the ancillary imaging features from any of the two groups.
9-
11 The present study identified that the updated 2022 KLCA-NCC imaging criteria on ECA-MRI significantly improve the sensitivity of “definite” or “probable” HCC categories compared with the 2018 KLCA-NCC but not on HBA-MRI. This may be explained by the fact that APHE is known to be better depicted on ECA-MRI than on HBA-MRI because of the relatively higher gadolinium dosage and lower incidence of motion during the AP of ECA-MRI. Nevertheless, the updated 2022 KLCA-NCC provided comparable diagnostic performances in “definite” or “probable” HCC categories between ECA-MRI and HBA-MRI, showing sensitivities of over 83% and specificities of over 90%.
Our study has some limitations. First, there may have been an inevitable selection bias owing to the retrospective nature of the study. Second, this study was performed in an area endemic to hepatitis B viral infection; therefore, the generalizability of the results may be limited. Third, the diagnostic performances of ECA-MRI and HBA-MRI were not compared intra-individually. Owing to the retrospective nature of this study, it was not possible to perform MRI using both contrast agents. Instead, two types of contrast agents were available at our institution, and referring physicians were encouraged to select one type according to their judgment. Based on the available data, we included a substantial number of patients at high risk for HCC using each contrast agent and compared the diagnostic performances between the two independent ECA-MRI and HBA-MRI groups. Nonetheless, the choice of MRI contrast agent may have introduced potential selection bias. Fourth, patients with multiple hepatic lesions were included in this study; however, no adjustment was made for clustering effects. Finally, the use of consensus categorization may overestimate diagnostic performance compared with clinical single-reader scenarios.
In conclusion, HBA-MRI provides better sensitivity than ECA-MRI without compromising specificity in “definite” HCC category of both 2018 and 2022 KLCA-NCC criteria. On ECA-MRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC criteria may improve sensitivity in the diagnosis of HCC compared with the 2018 KLCA-NCC.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download