Abstract
Parenchymal-sparing hepatectomy (PSH), though technically challenging, is emerging as a choice of treatment for colorectal liver metastases (CRLM). PSH in Jehovah’s witness (JW) patients, for whom transfusion is not an option, involves complex surgical and medicolegal issues. A 52-year-old JW male with synchronous, multiple, bilobar liver metastases from a rectal adenocarcinoma was referred following neoadjuvant chemotherapy. At surgery, 10 metastatic deposits were observed and confirmed by intraoperative ultrasonography. Parenchymal-sparing non-anatomical resections were performed using a cavitron ultrasonic aspirator with the application of intermittent Pringle maneuvres. Histology confirmed multiple CRLMs with tumor-free resection margins. PSH is increasingly employed for CRLMs to preserve residual liver volume and minimize morbidity without compromising oncological outcomes. It is technically challenging, especially in the presence of bilobar, multi-segmental disease. This case illustrates the feasibility of performing complex hepatic surgery in special patient groups by meticulous planning and preparation involving multiple specialties and the patient.
Colorectal cancer is the third commonest malignancy worldwide, with a rising incidence in developing countries.1 Approximately 50% of patients will develop colorectal liver metastases (CRLMs).2 Resection offers the best chance of survival and cure for patients with CRLMs.3 Parenchymal sparing liver surgery is increasingly employed in CRLMs to achieve tumor clearance while preserving residual liver volume.2,4,5
Hepatic surgery has the potential for substantial blood loss that requires blood transfusion.6 This poses a challenge in Jehovah’s witness (JW) patients who abstain from transfusions of primary and some of the secondary blood components.
We describe a patient who underwent a parenchymal-sparing hepatectomy (PSH) without blood transfusions for multiple, bilobar CRLMs. This case report is described according to CARE guidelines (available at https://www.care-statement.org/).
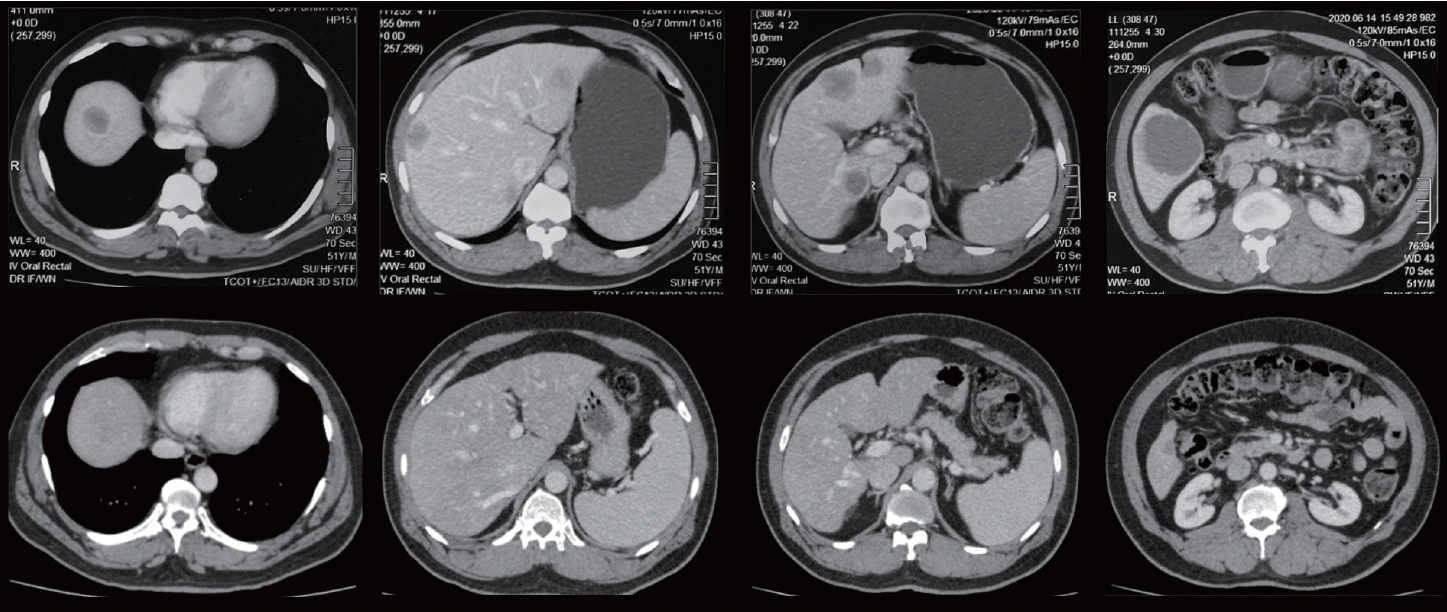
A 52-year-old American Society of Anesthesiologists physical status II, JW male with synchronous, multiple, bilobar CRLMs from a low rectal adenocarcinoma, expressing the wild-type KRAS genotype, was referred by the oncologist for surgical review. He was after eight cycles of neoadjuvant chemotherapy with FOLFOX and cetuximab (Fig. 1) and a favorable response had been noted in the restaging abdominal computed tomography (CT). There was a reduction in the number and size of the lesions with three lesions noted in segments IV (8×8 mm), VI (16×16 mm), and VIII (8×5 mm) in the restaging CT (Fig. 1).
Literature provided by the patient detailing JW restrictions on blood product use and alternative options were examined. It was also determined that his choices were made of free will without coercion. The patient and his family were counseled and consented by the surgical and anesthetic team on the potential for life-threatening hemorrhage during liver surgery without the option of red cell transfusion. The patient consented to transfusion of cryoprecipitate and albumin if required. His preoperative hemoglobin was 13.5 g/dL and his coagulation profile was normal.
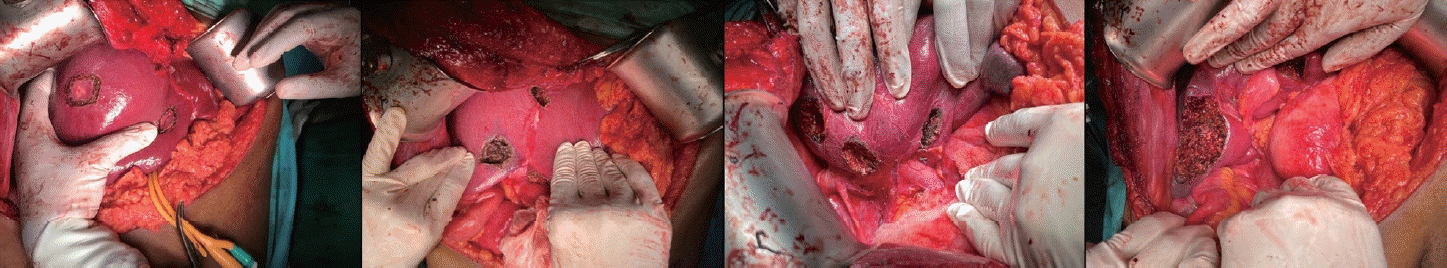
At surgery, 10 metastatic deposits demonstrating capsular cicatrization were observed in segments VIII (2), VII (2), VI (2), IVA (1), IVB (1) and III (2). Intraoperative ultrasonography (IOUS) confirmed these lesions and a normal residual liver. Parenchymal-sparing, non-anatomical resections (metastasectomies) were performed using the cavitron ultrasonic aspirator (CUSA) (Fig. 2). Intermittent Pringle maneuvers of 15 minutes with a 5-minute break for reperfusion were used during parenchymal transection. The total Pringle time was 120 minutes with an estimated blood loss of 1,550 mL. A low central venous pressure (CVP) was maintained during parenchymal transection. Bipolar diathermy and vascular clips were used for hemostasis over the transected liver surface. A cell saver was not available for use.
His postoperative hemoglobin was 9.6 g/dL and recovery, was complicated by a period of paralytic ileus. He was discharged on hematinics on the 9th postoperative day after complete recovery. The histology confirmed R0 resections of all resected CRLMs. He was referred back to the oncologist for neoadjuvant therapy of the primary, prior to rectal surgery. Following completion of neoadjuvant therapy and vaccination for COVID-19, he underwent resection of the rectal tumor 6 months from PSH. Restaging CT and intraoperative assessment demonstrated a tumor-free remnant liver.
Liver resection is the curative option for CRLMs and is aggressively pursued even in extensive bilobar disease, provided adequate residual liver is available. PSH is emerging as a therapeutic choice for CRLMs over conventional lobar or segmental resections since it offers comparable oncological outcomes with the added advantage of parenchymal preservation. This recognizes the importance of ‘what is left behind’ rather than ‘what is resected’ in liver surgery.
R0 resections, recurrence rates and overall and disease-free survival are similar between PSH and conventional resections.2,4,5 A 1 mm resection margin is considered oncologically adequate with wider margins conferring no added benefit.2,4,5 Tumor biology is increasingly recognized as a key predictor of recurrence than the margin status.2,4,5 Patients with the mutated KRAS genotype tend to have a poor prognosis and lower overall survival rates compared to ones with wild-type KRAS genotype regardless of the margin status.7
Moreover, PSH improves the opportunity of a salvage resection, with more remnant liver.2 It is utilized for bilateral and deep seated (>30 mm from the liver surface) liver lesions as well.2 Though associated with a longer operative times than conventional procedures, potential benefits observed include lower rates of estimated blood loss and intraoperative transfusions5 and lower rate of post-hepatectomy liver failure.5 In this patient, considering the number and distribution of metastases in the abdominal CT before neoadjuvant therapy, PSH was superior to a staged hepatectomy in terms of clearance and feasibility.
JW individuals abstain from transfusion of whole blood or primary blood components such as red cells, white cells, platelets and plasma, and some or all the secondary blood components.8 Pre-operative donation and auto-transfusion is not accepted as well. Autologous blood transfusion and intraoperative cell salvage is accepted, since they occur in continuity with the patient’s circulation.8
Optimization of pre-operative hemoglobin with hematinics and human recombinant erythropoietin, acute normovolemic hemodilution, autologous blood donation, intra-operative cell salvage, use of anti-fibrinolytic drugs and minimization of routine blood investigations reduce transfusion requirements.8,9
Maintenance of a low CVP (<5 cm H2O) and the use of inflow occlusion with an intermittent Pringle maneuver, reduces intra-operative blood loss. A case series of 15 hepatic resections done in JW patients demonstrated a median blood loss of 400 mL (range, 100-1,500). The higher than anticipated blood loss of 1,550 mL was mainly accounted for by an inadvertent retro-hepatic venous bleed.
Liver surgery in a JW poses unique clinical and ethical challenges. As was demonstrated in this case, meticulous planning and preparation that involves the surgical and anesthetic team leads to safe surgery and good outcomes. Counseling and involvement of the patient and the family in perioperative care is equally important to facilitate this process.
A key message illustrated by this case is the importance of respecting the autonomy and addressing the health needs of special patient groups, while overcoming therapeutic and ethical challenges.
Notes
Ethics Statement
This study was carried out in accordance with the principles of the Declaration of Helsinki. The patient has given consent for the publication of images and other relevant details.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

2. Moris D, Dimitroulis D, Vernadakis S, Papalampros A, Spartalis E, Petrou A, et al. Parenchymal-sparing hepatectomy as the new doctrine in the treatment of liver-metastatic colorectal disease: beyond oncological outcomes. Anticancer Res. 2017; 37:9–14.

3. Schadde E, Grunhagen DJ, Verhoef C, Krzywon L, Metrakos P. Limitations in resectability of colorectal liver metastases 2020 - a systematic approach for clinicians and patients. Semin Cancer Biol. 2021; 71:10–20.

4. Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, et al. Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J Gastrointest Surg. 2017; 21:1076–1085.

5. Deng G, Li H, Jia GQ, Fang D, Tang YY, Xie J, et al. Parenchymalsparing versus extended hepatectomy for colorectal liver metastases: a systematic review and meta-analysis. Cancer Med. 2019; 8:6165–6175.

6. Lim C, Salloum C, Esposito F, Giakoustidis A, Moussallem T, Osseis M, et al. Safety and feasibility of elective liver resection in adult Jehovah’s witnesses: the Henri Mondor Hospital experience. HPB (Oxford). 2018; 20:823–828.

7. Margonis GA, Sasaki K, Andreatos N, Kim Y, Merath K, Wagner D, et al. KRAS mutation status dictates optimal surgical margin width in patients undergoing resection of colorectal liver Metastases. Ann Surg Oncol. 2017; 24:264–271.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download