Abstract
Background/Aim
To evaluate the applicability of transarterial chemoembolization (TACE) treatment with doxorubicin drug-eluting beads (DEBs) in advanced hepatocellular carcinoma (HCC) patients with portal vein invasion (PVI).
Methods
This prospective study was approved by the institutional review board and informed consent was obtained from all participants. A total of 30 HCC patients with PVI received DEB-TACE between 2015 and 2018. The following parameters were evaluated: complications during DEB-TACE, abdominal pain, fever, and laboratory outcomes, including liver function change. Overall survival (OS), time to progression (TTP), and adverse events were also analyzed and assessed.
Results
DEBs measuring 100–300 μm in diameter were loaded with doxorubicin (150 mg per procedure). There were no complications during DEB-TACE and no significant differences in the levels of prothrombin time, serum albumin, or total bilirubin at follow-up compared to baseline. The median TTP was 102 days (95% confidence interval [CI], 42–207 days) and the median OS was 216 days (95% CI, 160–336 days). Three patients (10%) had severe adverse reactions, including transient acute cholangitis (n=1), cerebellar infarction (n=1), and pulmonary embolism (n=1), but no treatment-related death occurred.
Systemic therapies are regarded as a standard treatment for hepatocellular carcinoma (HCC) patients with portal vein invasion (PVI).1,2 However, sorafenib only showed a limited survival benefit over placebo in advanced HCC patients with macroscopic vascular invasion in the SHARP trial.3 In a recent study, although the combination of atezolizumab and bevacizumab was associated with better clinical outcomes over sorafenib, only 38% of patients in the combination group had macrovascular invasion.4 Asian HCC practice guidelines5 recommend conventional transarterial chemoembolization (TACE) for HCC patients with lobar PVI. Moreover, in real world practice, conventional/drug-eluting bead (DEB) TACE, radioembolization, external radiation therapy, arterial or systemic chemotherapy, and combination therapy are all widely performed on patients with PVI, depending on the practitioner’s preference. Recently, a randomized clinical trial reported that conventional TACE plus radiotherapy results in an improved survival period compared to sorafenib treatment for advanced HCC patients with macrovascular invasion.6
In many studies, DEB-TACE has shown non-inferior survival for HCC patients with no significant differences in systemic toxicity compared to conventional TACE.7,8 Moreover, DEB-TACE has also shown better results in post-embolization syndrome.9,10 Therefore, in many HCC practice guidelines, including the European Association for the Study of the Liver (EASL),11,12 DEB-TACE is recognized as an equivalent treatment modality to conventional TACE.
The efficacy and safety of conventional TACE in HCC patients with PVI has been widely reported in the literature.13-15 However, the survival advantages and safety of DEB-TACE have not been demonstrated in patients with advanced HCC with PVI. In this prospective study, the safety and survival advantages of DEB-TACE in patients with advanced HCC and PVI were evaluated.1,16
Patients with PVI, from the third or lower order branch (Vp1) to main portal vein (Vp4), who were diagnosed with HCC and received DEB-TACE as initial treatment were eligible for this study. The diagnosis of HCC was based on the non-invasive criteria of the American Association for the Study of Liver Diseases (AASLD).17 PVI was deemed to be present on observation of a low-attenuating mass that expanded the portal vein and/or filling defects in the main portal vein on three-phase dynamic computed tomography (CT) and/or magnetic resonance imaging (MRI). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed (Supplementary Table 1).
Informed consent was obtained from all patients prior to enrollment. The study was designed by the principal academic investigators. Data were managed in parallel by the Data and Safety Monitoring Board and the principal investigators. Clinical research information is available on the International Clinical Trials Registry Platform website, which is provided by the World Health Organization.
Subjects who were eligible for inclusion/exclusion criteria after the screening test received the first DEB-TACE treatment within a maximum of 28 days after the screening test. The next DEB-TACE was performed based on the re-performing criteria, and the treatment period was considered from the first DEB-TACE to the last treatment. At baseline, abdominal and chest imaging (dynamic CT and/or MRI) were performed within 28 days before the first DEB-TACE. The efficacy and safety of DEB-TACE was evaluated every 4 weeks after each DEB-TACE treatment period. Additional DEB-TACE was performed as needed within 4–6 weeks of the previous DEB-TACE. Patients who did not require any additional DEB-TACE due to absence of residual tumor after DEB-TACE were clinically evaluated every 4 weeks and received dynamic spiral CT or MRI every 8 weeks. Subjects who met the DEB-TACE treatment discontinuation criteria were confirmed safety within 30 days (±7 days) of their last DEB-TACE treatment and had a follow-up period for survival analysis every 84 days (±7 days) (84 days based on DEB-TACE).
Data regarding clinical and laboratory findings were prospectively acquired from all subjects by reviewing their electronic medical records. One radiologist (H.C.K) who was blinded to the survival data independently reviewed all the radiologic images to determine the number and size of tumors, the presence or absence of vascular invasion, the treatment response, and recurrence. Diffuse or infiltrative type HCC with no discrete margin was regarded as a large mass, and in this case the radiologist measured the size of diffuse or infiltrative type of tumor.18 Based on the CT or magnetic resonance imaging findings, the radiologist identified the border of tumor and non-tumor parenchyma and measured the size of tumor, as previously described.19
DEB-TACE was performed if the patient agreed to undergo the procedure. DEB (DC bead, Biocompatibles, Farnham, United Kingdom) measuring 100–300 µm in diameter were loaded with doxorubicin according to the manufacturer’s instructions (150 mg per procedure). DEB-TACE was performed as selectively as possible through the lobar, segmental, or subsegmental arteries depending on the tumor distribution and hepatic functional reserve. Two months later, a three-phase dynamic CT scan with contrast material was conducted to identify any remaining viable or recurring tumors. If the patient’s hepatic functional status remained poor (Child-Pugh class B or higher) and there was no evidence of hepatic decompensation (such as uncontrolled ascites or portosystemic encephalopathy), DEB-TACE was performed until there was no sign of viable intrahepatic tumors. The absence of viable intrahepatic tumors was determined by observing a decrease in parenchymal and intraportal tumor thrombi, as well as the absence of enhancing tumors on three-phase dynamic CT scans and the lack of tumor staining during follow-up hepatic arteriography.20 Catheterization via a femoral artery and superselective embolization of the hepatic artery branches feeding the tumor were performed.
The primary endpoint was time-to-progression (TTP), defined as the time from the date of DEB-TACE to time of tumor progression. The secondary endpoint included overall survival (OS), defined as the interval between the date of diagnosis and the date of death from any cause, progression free survival (PFS), defined as the interval between the date of DEB-TACE and disease progression or death from any cause, and treatment-related adverse events, defined as any event not present prior to the initiation of the treatments or any event already present that worsens in either intensity or frequency following exposure to the treatments. Tumor progression after DEB-TACE was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).21
The baseline patient characteristics were expressed as the mean (±standard deviation) or median (range). The KaplanMeier method was used to estimate the OS and TTP. Multivariate Cox proportional hazards regression analysis was performed to identify the prognostic factor for OS and TTP. All data obtained from subjects administered drugs for clinical study at least once were included in the intention-to-treat (ITT) analysis. Both the safety and efficacy assessments were analyzed by ITT analysis group. All statistical analyses were performed using PASW statistics for Windows, ver. 22.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were twosided and conducted in an explorative manner with a significance level of P<0.05.
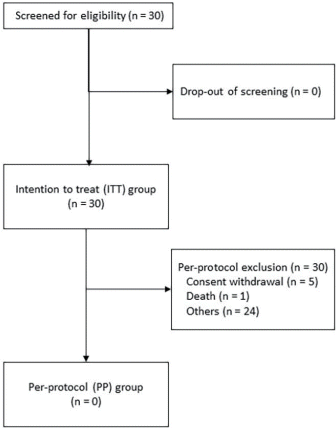
A total of 30 patients (26 males [86.67%] and four females [13.33%]) with HCC and PVI treated between October 2015 and September 2018 at Seoul National University Hospital were enrolled in this exploratory study, and those who dropped out were not replaced. DEB-TACE and evaluation were performed following the protocol. Patient flow from registration to analysis is summarized in the CONSORT diagram of patient flowchart (Fig. 1). Table 1 shows the baseline characteristic of the study population. The mean age of the patients was 59.7 years (range, 39–77). Hepatitis B virus (HBV) (21 [70.0%]) was the most common etiology of HCC. All patients were classified as Child-Pugh class A (25 [83.3%]) and B (5 [16.6%]). The median serum alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence-II (PIVKA-II) levels were 274.30 ng/mL (range, 3–100,500) and 502.5 mAU/mL (range, 15–75,000), respectively. Sixteen patients (53.3%) had diffuse or infiltrative type HCC, 18 (60.0%) had bilobar tumor extent, and 15 (50.0%) patients had main PVI (Vp4).
No complications occurred during DEB-TACE. The clinical and biochemical toxicity is summarized in Table 2. Treatment-emergent adverse events (TEAEs) occurred in 20 patients (66.67%) and three patients (10.00%) had serious adverse reactions including cholangitis (n=1), cerebellar infarction (n=1), and pulmonary embolism (n=1), in the ITT analysis group during the study period. One event with cerebellar infarction was considered “probable relevance” with DEB-TACE and two events were considered “not related” with DEB-TACE. One patient with cholangitis was treated with intravenous antibiotic therapy but did not receive biliary drainage because of a lack of bile duct dilatation. There were no DEB-TACE-related deaths. The frequency of postembolization syndrome was significantly higher in patients with Vp4 than in patients with Vp1–3 (10 [66.67%] vs. 5 [33.33%], respectively; P=0.0029). However, there was no hepatic failure in our patients.
The results for tumor response are summarized in Table 3. There was no complete response, but partial response (PR) and stable disease (SD) were achieved in eight (26.67%) and 10 progressive disease (PD) patients (33.33%). Seven patients (23.33%) had PD after DEB-TACE.
Among the 30 patients in the ITT analysis group, tumor progression or death was confirmed in 23 patients (76.67%). The median TTP was 102.00 days (range, 42.00–207.00) (Fig. 2A) and the median survival time of PFS was 167.00 days (range, 61.00–248.00) (Fig. 2B). As a result of OS, death was confirmed in 21 patients (70.00%) out of 30 patients, with a median OS of 216.00 days (range, 160.00–336.00) (Fig. 2C). Multivariate Cox regression analysis showed that the baseline PVI Vp4 level (vs. Vp1–3 level) was not a significant prognosticator for OS (hazard ratio [HR], 0.399; 95% confidence interval [CI], 0.026–6.198; P=0.511). Moreover, multivariate Cox regression analysis demonstrated that HBV etiology (HR, 5.79; 95% CI, 1.131–29.652; P=0.035) was a significant risk factor for poor survival, and hepatitis C virus etiology (HR, 26.304; 95% CI, 0.749–923.218; P=0.072) and tumor number (3 or more vs. 1–2) (HR, 0.053; 95% CI: 0.002–1.701; P=0.097) had a tendency to be associated with poor survival (Table 4).
Additionally, survival analyses were performed between the disease control group (PR+SD) and the other group (PD+not applicable) according to treatment response (Table 5). The disease control group was found to have a significantly longer TTP than the other group (median, 207 days [95% CI, 57–207] vs. 35 days [95% CI, 22–42]; P<0.0001) (Fig. 3A). PFS was significantly longer in the disease control group compared to the other group (median, 207 days [95% CI, 102–367] vs. 41 days [95% CI, 28–248]; P=0.0037) (Fig. 3B). OS was significantly longer in the disease control group compared to the other group (median, 224 days [95% CI, 131–389] vs. 176 days [95% CI, 80–260]; P=0.0487) (Fig. 3C). However, no significant different clinical outcomes were observed in terms of OS, PFS, and TTP between Child-Pugh class A and B. The OS was similar with Child-Pugh class A to Child-Pugh class B (median, 216 days [95% CI, 160–260] vs. 389 days [95% CI, 62–399]; P=0.6264). PFS was similar with Child-Pugh class A to Child-Pugh class B (median, 167 days [95% CI, 57–336] vs. 131 days [95% CI, 41–389]; P=0.8805). TTP was similar with Child-Pugh class A to Child-Pugh class B (median, 102 days [95% CI, 42 to not reached] vs. 207 days [95% CI, 41–207]; P=0.3954).
This is the first prospective study in which the safety and efficacy of DEB-TACE for the treatment of advanced HCC with PVI is evaluated. In this study, DEB-TACE was safe but achieved modest survival gain in patients with advanced HCC and PVI. Although the baseline PVI level was not a significant survival factor, tumor number had a tendency to be associated with overall survival. Based on the results, DEB-TACE may be a treatment option for patients with HCC and PVI <3.
Many international guidelines recommend only systemic therapy using molecular targeting agents or immune checkpoint inhibitors for the treatment of advanced HCC. Recently, the combination of atezolizumab and bevacizumab has been demonstrated to result in significantly longer OS and PFS, as well as better patient-reported outcomes, than sorafenib as a first-line systemic therapy.4 However, approximately 30% of patients with HCC do not respond to these combination therapies. Therefore, there is a need to establish a more effective therapy than combination of atezolizumab and bevacizumab for the treatment of HCC.
Uka et al.22 reported that most patients with an advanced intrahepatic tumor stage and major vascular invasion died from intrahepatic tumor. The cause of death for patients with main PVI is the progression of intrahepatic HCC.23 Therefore, providing locoregional strategies for the control of intrahepatic tumors is a reasonable research aim. Recent data have shown that DEB-TACE may be an effective locoregional approach by improving drug delivery, reducing systemic drug exposure, and decreasing adverse effects.24-26 Moreover, a retrospective study demonstrated the safety and effectiveness of DEB-TACE in patients with advanced HCC.27 DEBs are an innovative system for delivering drugs through embolization, which aims to provide a higher and more consistent release of the drug directly to the tumor, while limiting the amount of drug released into the wider bloodstream. The ultimate goal is to enhance the drug’s response to the tumor while reducing its toxicity in other parts of the body. Research conducted both in laboratories and with patients has demonstrated that DEB-TACE enables higher and longer retention of doxorubicin within the tumor, while also resulting in lower levels of the drug circulating throughout the body, when compared to conventional TACE methods.24,28-30
Although TACE has been contraindicated in HCC patients with main PVI, occlusion of the main portal vein induces the gradual development of periportal collateral circulation. In addition, the superselective catheterization of feeder vessels with a microcatheter helps minimize hepatic parenchymal damage during TACE. Therefore, if a superselective TACE technique is used in patients with HCC invading the main portal vein, the occurrence of fatal complications appears unlikely, especially in patients with well-developed collateral circulation around the portal trunk and a good hepatic reserve.31 Although the median OS of DEB-TACE in our study was only 216 days, which was not significantly higher than those of systemic therapies including sorafenib, lenvatinib, and atezolizumab+bevacizumab, DEB-TACE could be performed safely in patients with PVI. Thus, DEB-TACE could be considered a potential combinatorial locoregional therapy for use as a systemic therapy.
This study has several limitations. First, a relatively small sample size was used. Further studies using a larger sample size will be needed to confirm the small differences observed in survival among the treatment groups. Second, the efficacy of DEB-TACE for patients with PVI was not compared with that of conventional TACE in this study. DEB-TACE may induce peripheral and permanent embolization, which may lead to wider hepatic infarction than conventional TACE. Therefore, further studies evaluating the efficacy and safety of DEB-TACE compared to conventional TACE are needed.
In conclusion, for patients with HCC with PVI >3, DEB-TACE showed relatively longer OS with relatively lower incidence of treatment-related adverse events. Therefore, DEBTACE may be a treatment option for patients with advanced HCC and PVI. Further larger scale prospective studies will be needed to confirm the efficacy of DEB-TACE in these patients, as well as to compare the therapeutic efficacy of DEB-TACE with newer systemic therapies.
Notes
Ethics Statement
All patients provided written informed consent before enrollment. The study protocol and procedures were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. D-1703-023-836). All methods and procedures associated with this study were conducted in accordance with the good clinical practice guidelines and ethically with the principles of the Declaration of Helsinki and local law.
Funding Statement
This work was supported by the Liver Research Foundation of Korea. This study was supported by Biocompatibles UK Ltd.
Supplementary Material
Supplementary data can be found with this article online https://doi.org/10.17998/jlc.2023.02.08.
References
1. Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008; 48(Suppl 1):S20–S37.

2. Schwartz JD, Beutler AS. Therapy for unresectable hepatocellular carcinoma: review of the randomized clinical trials-II: systemic and local non-embolization-based therapies in unresectable and advanced hepatocellular carcinoma. Anticancer Drugs. 2004; 15:439–452.

3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.

5. Korean Liver Cancer Study Group; National Cancer Center. 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522.
6. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.

7. Gorodetski B, Chapiro J, Schernthaner R, Duran R, Lin M, Lee H, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017; 27:526–535.

8. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011; 22:1545–1552.

9. Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C, Dueber C, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015; 15:465.

10. Xie ZB, Wang XB, Peng YC, Zhu SL, Ma L, Xiang BD, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015; 45:190–200.

11. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943.
12. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.

13. Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011; 258:627–634.

14. Li MF, Leung HW, Chan AL, Wang SY. Network meta-analysis of treatment regimens for inoperable advanced hepatocellular carcinoma with portal vein invasion. Ther Clin Risk Manag. 2018; 14:1157–1168.

15. Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011; 18:413–420.

16. Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010- 2016. Clin Mol Hepatol. 2016; 22:7–17.

17. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.

18. Kneuertz PJ, Demirjian A, Firoozmand A, Corona-Villalobos C, Bhagat N, Herman J, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012; 19:2897–2907.

19. Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015; 35:371–386.

20. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.

21. Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017; 66:1166–1172.

22. Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007; 13:414–420.

23. Lee HS. Management of patients with hepatocellular carcinoma and extrahepatic metastasis. Dig Dis. 2011; 29:333–338.

24. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12:2563–2567.

25. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007; 46:474–481.

26. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–52.

27. Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014; 37:381–387.

28. Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006; 17:335–342.

29. Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, et al. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007; 18:1691–1699.

Figure 2.
Kaplan-Meier curves of (A) time to progression, (B) progression-free survival, and (C) overall survival.
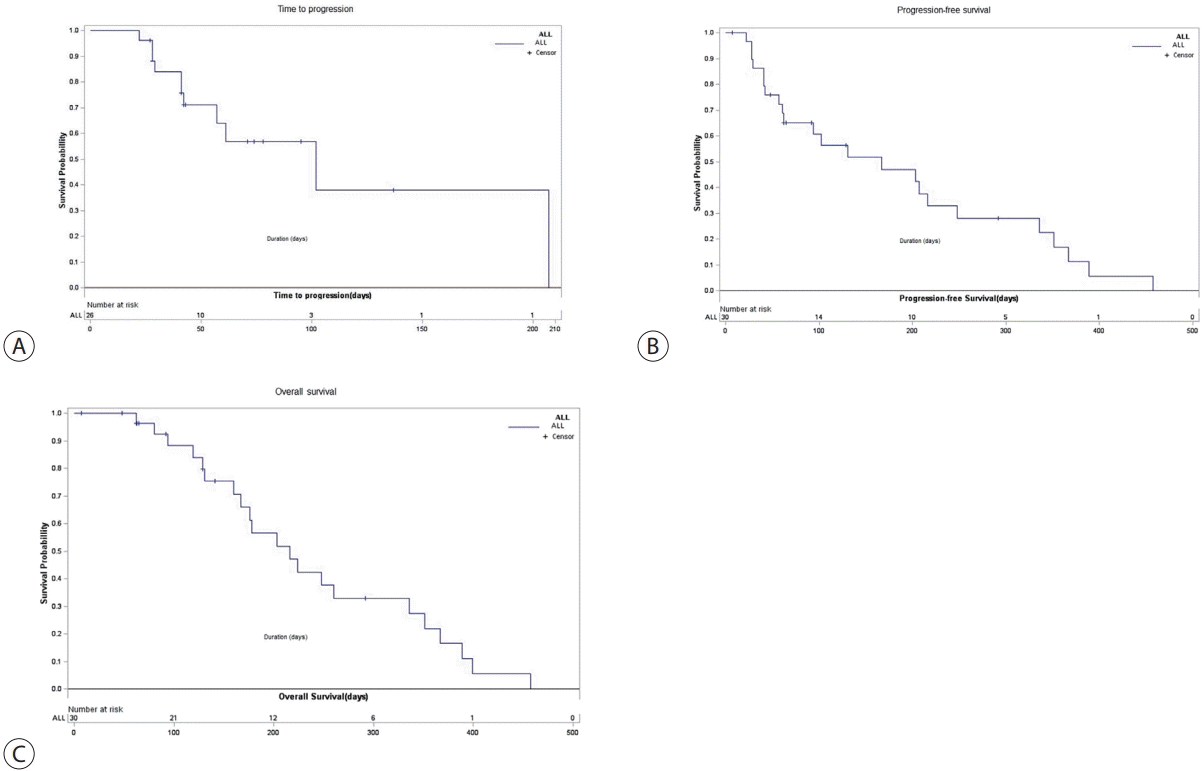
Figure 3.
Kaplan–Meier curves of (A) time to progression, (B) progression-free survival, and (C) overall survival according to subgroup analysis (the disease control group [PR+SD] vs. the other group [PD+not applicable]). PD; progressive disease, PR; partial response, SD; stable disease.
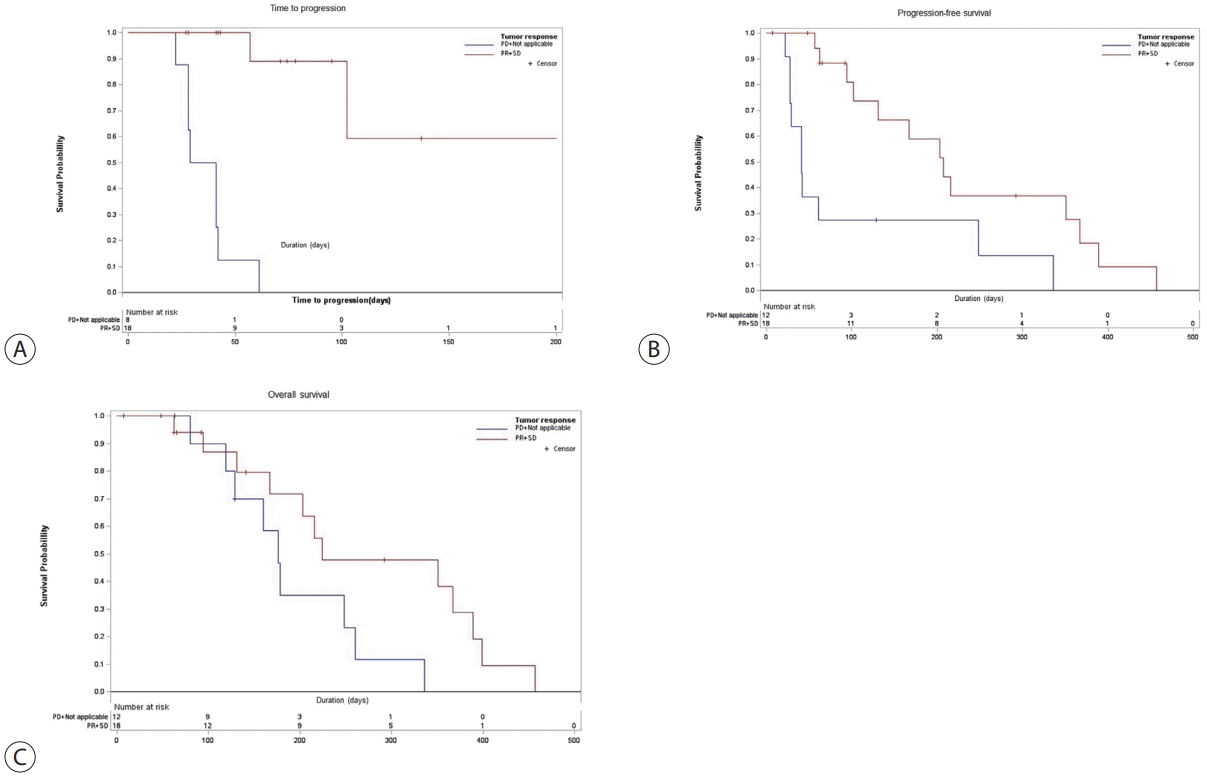
Table 1.
Baseline characteristics
Table 2.
Toxicity from DEB-TACE
Table 3.
Baseline characteristics
| Variable | Value |
|---|---|
| Complete response | 0 (0.00) |
| Partial response | 8 (26.67) |
| Stable disease | 10 (33.33) |
| Progressive disease | 7 (23.33) |
| Not applicable | 5* (16.67) |
| Total | 30 (100.00) |
Table 4.
Cox regression analyses to predict risk factors for survival
Table 5.
Survival analyses according to treatment response
|
DC bead |
P-value | ||
|---|---|---|---|
| PR+SD | PD+not applicable | ||
| ITT | 18 | 12 | |
| Time to progression (days) | 207 (57–207) | 35 (22–42) | <0.0001* |
| Progression in HCC | 18 | 8 | |
| Yes | 3 (16.67) | 8 (100.00) | 0.0001† |
| No | 15 (83.33) | 0 (0.00) | |
| Overall survival rate (days) | 224 (131–389) | 176 (80–260) | 0.0487* |
| Survival rate | 18 | 12 | |
| Yes | 12 (66.67) | 9 (75.00) | 0.7036† |
| No | 6 (33.33) | 3 (25.00) | |
| Progression-free survival | 18 | 12 | |
| Yes | 13 (72.22) | 10 (83.33) | 0.6693† |
| No | 5 (27.78) | 2 (16.67) | |
| Time to progression free survival (days) | 207 (102–367) | 41 (28–248) | 0.0037 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download