Abstract
Purpose
We investigated the effects of economic status (classified based on insurance type and residential area) on oncological outcomes of prostate cancer using a nationwide database. We additionally investigated oncological outcomes based on economic status and residential area in patients who underwent surgical treatment.
Materials and Methods
The study included 75,518 men with newly diagnosed prostate cancer between 2009 and 2018 in whom oncological outcomes were investigated based on economic status and residential area. Among the 75,518 men with prostate cancer, the data of 29,973 men who underwent radical prostatectomy were further analyzed. Multivariate analysis was performed to determine the effects of economic status and residential area on postoperative oncological outcomes.
Results
Among the 75,518 patients with prostate cancer, 3,254 (4.31%) were medical aid beneficiaries. The 5-year overall survival rates were 81.2% and 64.8% in the health insurance and medical aid groups, respectively. Radical prostatectomy was more common in the health insurance group, and surgical intervention was significantly affected by the residential area. Among patients who underwent surgery, 5-year androgen deprivation therapy–free and overall survival were better in the health insurance group. Multivariate analysis showed that insurance type and residential area were significantly associated with the androgen deprivation therapy–free and overall survival after adjustment for other variables.
Prostate cancer is the second most common solid tumor worldwide [1] and the most commonly diagnosed non-skin cancer in the United States [2]. However, reportedly, prostate cancer shows relatively favorable oncological outcomes compared with those of other cancers [3], and per the US Preventive Services Task Force (USPSTF) statement in 2012, there is moderate certainty that the benefits of prostate-specific antigen (PSA) screening do not outweigh the harms; therefore, The USPSTF recommends against PSA screeening [4]. However, following this proposal, the percentage of patients with advanced prostate cancer has increased slightly in the United States [5]. Therefore, the USPSTF, recently, recommendation statement proposes individualized decision-making for prostate cancer screening after sufficient discussion [6]. Prostate cancer is the 4th most common solid cancer in Korean men, with a rapid increase in incidence [3]. However, PSA testing is not included in the public health checkup program, despite the steady increase in prostate cancer mortality rates [7].
Several factors are known to affect the therapeutic approach to and oncological outcomes of prostate cancer. Although personal health status, age, tumor aggressiveness, and tumor stage are important, these factors are unmodifiable variables. PSA screening may be effective for early detection of prostate cancer and serves as an easy and cost-effective tool to improve oncological outcomes in at least some patients [6]. In essence, the benefits and scope of the public health checkup program could be further enhanced to detect prostate cancer and improve survival if it is possible to select men who are likely to benefit from public health checkup programs that include regular PSA screening.
In Korea, PSA testing is affected by the socioeconomic status or medical accessibility because PSA screening is not included in public health checkup programs. Moreover, not only selection of the treatment method but even post-treatment oncological outcomes may be affected by socioeconomic status and/or medical accessibility, which cause a medical imbalance with regard to prostate cancer management, as reported by previous studies [8,9]. However, no large-scale Korean study has discussed this subject.
Using a nationwide database provided by the Health Insurance Review and Assessment Service (HIRA), we investigated the role of economic status and residential area as contributors to the differences in treatment methods and oncological outcomes in patients with newly diagnosed prostate cancer. We specifically investigated the effects of economic status and residential area on oncological outcomes in patients with prostate cancer who underwent surgical treatment.
This study included 118,749 men who visited medical facilities with a diagnosis of prostate cancer between 2007 and 2018; we analyzed data obtained from the HIRA database. Exclusion criteria were as follows: (1) Diagnosis of prostate cancer between 2007 and 2008 (31,308 men; we only selected patients with newly diagnosed prostate cancer). (2) Unavailability of data regarding the exact timing of prostate cancer diagnosis (4,668 men). (3) Treatment received for prostate cancer before prostate cancer diagnosis and delay of at least 6 months until treatment after diagnosis (3,502 men) [10]. These patients were excluded to ensure accurate assessment of oncological outcomes in patients who underwent surgery. (4) Administration of preoperative neoadjuvant hormone therapy or chemotherapy (2,025 men). After excluding 1,728 veteran patients with prostate cancer, 75,518 patients with prostate cancer were initially included in the analysis. We further analyzed data of 29,973 patients with prostate cancer who underwent surgery, to compare oncological outcomes after radical prostatectomy. The current study was approved by the Institutional Review Board of our hospital.
All diseases were defined based on the Korean Classification of Diseases, 7th Revision codes, a modification of the International Classification of Diseases, 10th Revision. Patients with prostate cancer were defined as men who visited medical facilities and were assigned diagnostic code C61. Hypertension was defined using codes I10–I15 and diabetes using codes E10–E14 [11]. A ‘residential area’ associated with prostate cancer was defined as the geographical location at which the prostate cancer diagnosis code was initially registered. Regions were re-categorized into six subareas (Seoul: Seoul vs. Gyeonggi: Gyeonggi province and Incheon vs. Gyeongsang: Gyeonsang province, Busan, Daegu, and Ulsan vs. Jeolla: Jeolla province, Gwangju, and Jeju island vs. Chungcheong: Chungcheong province and Daejeon vs. Gangwon: Gangwon province). Presence of surgery and the type of surgery (open vs. robotic) were determined using operation, anesthesia, and pathology codes [12]. The interval between diagnosis and surgery was determined using the date of C61 code registration and the date of surgery. The timing of androgen-deprivation therapy (ADT) was determined using the Anatomical Therapeutic Chemical Classification System codes, and ADT-free survival was calculated. The timing of death was determined based on absence of clinic visits over at least 1 year.
The study cohort was categorized into two groups based on insurance type (health insurance vs. medical aid group). The medical aid system is a public medical assistance program that provides healthcare benefits to low-income individuals; 3.0% of the entire Korean population is covered under this system [13]. Patient characteristics are expressed using the mean±standard deviation for continuous variables and frequencies and percentages for categorical variables. The percentages of patients who underwent radical prostatectomy and robotic surgery were recorded based on the residential area. Overall survival of the entire cohort was compared using Kaplan-Meier analysis based on insurance type and residential area.
We further analyzed the data of patients who underwent radical prostatectomy to compare postoperative oncological outcomes and analyzed patient characteristics based on insurance type. Kaplan-Meier analysis was performed to determine ADT-free survival and overall survival after radical prostatectomy and compared based on insurance type and residential area. Univariate and multivariate analyses were performed to confirm the variables associated with ADT-free survival and overall survival after surgical treatment. Variables that showed p < 0.2 on univariate analysis were subjected to multivariate analysis. All statistical analyses were performed using the SAS software ver. 9.4.2 (SAS Institute, Cary, NC) and the R software ver. 3.4.2 (http://www.r-project.org). A p-value < 0.05 was considered statistically significant.
Of the 75,518 patients with prostate cancer, 3,254 (4.31%) were included in the medical aid group (Table 1). Mean age was 72.2 and 68.6 years in the medical aid and health insurance groups, respectively (p < 0.001) and both hypertension and diabetes are more common in medical aid group (p < 0.001 for both). Radical prostatectomy was performed in 29,973 patients (37.7%) and was more frequently performed in the health insurance group (40.5% vs. 21.9%). The percentage of patients who underwent radical prostatectomy and robotic surgery was higher in metropolitan areas (Fig. 1). The 5-year overall survival rate was 80.5% (95% confidence interval [CI], 80.2 to 80.8) in the entire cohort. The 5-year overall survival was 81.2% (95% CI, 80.9 to 81.5) in the health insurance and 64.8% (95% CI, 63.1 to 66.6) in the medical aid group (Fig. 1). The 5-year survival rates were highest in Seoul (84.0%), followed by Gyeonggi (81.0%), Gyeongsang (79.6%), Chungcheong (78.4%), Jeolla (77.3%), and Gangwon (77.2%).
Among the 29,973 patients who underwent radical prostatectomy, 712 (2.38%) belonged to the medical aid group; patients in the health insurance group were younger than those in the medical aid group (66.6 vs. 69.4 years, p < 0.001). Although robotic surgery was performed less frequently in the medical aid group (35.5% vs. 57.8%), no difference was observed in the interval between diagnosis and surgical treatment. Robotic surgery was more frequently performed in metropolitan areas (Fig. 2). Although robotic surgery was more commonly performed in metropolitan areas in the health insurance group (p < 0.001), we observed no difference in the percentage of robotic surgery based on residential area in the medical aid group (p=0.690) (S1 Table).
The 5-year ADT-free and overall survival rates were 75.5% (95% CI, 75.1 to 76.1) and 91.7% (95% CI, 91.4 to 92.0), respectively among all patients who underwent radical prostatectomy. The 5-year ADT-free survival rate was 75.6% (95% CI, 75.1 to 76.1) in the health insurance group and 69.6% (95% CI, 66.2 to 73.2) in the medical aid group (Fig. 3). The 5-year overall survival was 92.0% (95% CI, 91.6 to 92.3) in the health insurance group and 80.8% (95% CI, 77.8 to 83.9) in the medical aid group. The 5-year ADT-free survival (Seoul: 78.2% vs. Gyeonggi: 75.8% vs. Chungcheong: 72.0% vs. Gangwon: 76.0% vs. Jeolla: 69.1% vs. Gyeongsang: 71.3%) and overall survival (Seoul: 92.9% vs. Gyeonggi: 91.1% vs. Chungcheong: 91.6% vs. Gangwon: 91.3% vs. Jeolla: 87.4% vs. Gyeongsang: 90.0%) also differed significantly based on residential area.
Univariate analysis showed that insurance type and residential area were significantly associated with ADT-free and overall survival, in addition to age as well as hypertension and diabetes. Multivariate analysis showed that insurance type was significantly associated with poor ADT-free survival (hazard ratio [HR], 1.203; p=0.008) and poor overall survival (HR, 1.778; p < 0.001) (Table 2). Additionally, residential area was significantly associated with ADT-free and overall survival.
Reportedly, prostate cancer shows relatively good oncological outcomes [3]; however, similar to other cancers, tumor stage is the most important determinant of oncological outcomes [14]. Therefore, early detection is important to improve post-treatment oncological outcomes, although overdiagnosis of prostate cancer based on widespread popularity of PSA screening is a potential disadvantage [15]. The current USPSTF recommendation statement proposes individualized decision-making regarding prostate cancer on case-by-case basis [6], and the European Association of Urology recommends a risk-adapted approach for PSA screening to improve oncological outcomes [16]. Notably, PSA screening is not included in the public health checkup program in Korea, and individuals and clinicians are responsible for prostate cancer diagnosis. Socioeconomic status and medical accessibility tend to affect the timing of prostate cancer diagnosis. In the current study, we investigated the effects of economic status and residential area on treatment and its outcomes, particulary after surgical therapy.
The current study showed that the overall 5-year survival rate of patients with newly diagnosed prostate cancer was 80.5%, although overall survival was only 64.8% in the medical aid group. Although the overall survival rate may appear exaggerated owing to the operational definition of survival used in this study, we observed a significantly high intergroup difference in survival rates. Previous studies have also reported that low socioeconomic status was associated with an increased risk of mortality in prostate cancer [17,18]. Therefore, men of low socioeconomic status need political support to improve survival, and PSA screening tests performed every 2–5 years included in the public health checkup in the medical aid program may serve as a feasible and reliable option. Although tumor stage was not included in the current study, approximately 70%–80% of patients with localized prostate cancer tended to undergo surgical therapy [19]. Moreover, usually, only localized cancer is considered an indication for surgical treatment in patients with prostate cancer [20], and postsurgical oncological outcomes were shown to be signficantly associated with the tumor stage. In the current study, although the difference between overall survival was reduced, economic status was significantly associated with ADT-free and overall survival after adjustment for other variables. Therefore, a low percentage of surgical treatment administered to the medical aid group and poor postoperative oncological outcomes may indicate delayed diagnosis of prostate cancer. Furthermore, a higher stage of prostate cancer is known to be associated with an increased cost burden [21]; therefore, early detection of this malignancy is important from the patient’s viewpoint, particularly for patients of low socioeconomic status (beneficiaries of the medical aid program).
In this study, the oncological outcomes of newly diagnosed prostate cancer were better in metropolitan areas, including in Seoul and Gyeonggi; these results are similar to those reported by previous studies [22,23] and may be attributable to the active implementation of private health checkup programs, including PSA screening tests in metropolitan areas [24–26], which facilitates early diagnosis of prostate cancer in these areas. Similarly, robotic surgery not covered by the public insurance program is also performed more frequently in metropolitan areas. In this study, non-metropolitan areas showed poor oncological outcomes, which might be attributable to the regional healthcare environment, including distance from the metropolitan area and from large regional cities, although further studies are required to conclusively determine the contributors to these findings. Interestingly, Chungcheong province, follwed by Gangwon provice, showed the worst oncological outcomes after srugery for prostate cancer. Considering the supeiror oncological outcomes of metropolitan area and the accessibility to metropolitan are from these provinces, it is possible that early prostate cancer patients dectected through health check-up who lived in these provinces received the further treatment in the metropolitan area. In addition, at 2017, there was only four tertiary hospitals in Chungcheong province and one tertiary hostpial in Gangwon province, respectivley. Moreover, a tertiay hospital with more than 1,000 beds were only one of them. Although these could be reason for the differences in oncological outcomes accroding to regions, standardized treatment strategies are reportedly associated with similar oncological outcomes regardless of regional differences [27]; therefore, it is important to establish a uniform therapeutic approach for management of prostate cancer.
Following are the limitations of this study. (1) We used operational definitions in this study. (2) Several treatment approaches, including active surveillance and radiotherapy were not analyzed; therefore, accurate prediction of the overall status of prostate cancer treatment and its outcomes was not possible in this study. (3) Several important clinical and pathologic variables which related to oncological outcomes, such as PSA level, clinical stage, tumor aggressiveness, and pathologic stage were unavailable in the HIRA database and were therefore not analyzed. (4) A residential area was defined as the first location at which prostate cancer diagnosis was registered; therefore, the actual address of patients was not confirmed, although prostate cancer diagnosis tends to be performed in areas in close proximity to patients’ residence. To our knowledge, this is the first study that investigated the effects of economic status and residential area on treatment patterns and oncological outcomes of prostate cancer in Korea. Moreover, we focused on oncological outcomes following prostate cancer surgery. This study highlights the effects of socioeconomic status and residential area on the diagnosis, treatment, and oncological outcomes of prostate cancer; therefore, in our view, the study makes a significant contribution to the literature and can provide useful guidelines to develop medical-related policies for prostate cancer diagnosis and treatment.
In conclusions, both the treatment pattern and oncological outcomes were shown to be associated with economic status and residential area. Political support is essential for early diagnosis and appropriate treatment of prostate cancer; medically vulnerable groups including men who receive medical aid support or those who reside outside metropolitan areas should undergo regular PSA screening tests.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The current study was approved by the Institutional Review Board of Boramae Medical Center (IRB No. 07-2019-32). The study was performed in accordance with the principles of the Declaration of Helsinki, and the requirement for informed consent was waived.
References
2. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012; 2012:152–6.

3. Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat. 2021; 53:316–22.

4. Moyer VA; US. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157:120–34.

5. Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017; 14:26–37.

6. Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 draft recommendation statement on screening for prostate cancer: an invitation to review and comment. JAMA. 2017; 317:1949–50.

7. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–50.

8. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013; 119:3067–75.

9. Gray PJ, Lin CC, Cooperberg MR, Jemal A, Efstathiou JA. Temporal trends and the impact of race, insurance, and socioeconomic status in the management of localized prostate cancer. Eur Urol. 2017; 71:729–37.

10. Xia L, Talwar R, Chelluri RR, Guzzo TJ, Lee DJ. Surgical delay and pathological outcomes for clinically localized high-risk prostate cancer. JAMA Netw Open. 2020; 3:e2028320.

11. Yoo S, Oh S, Park J, Cho SY, Cho MC, Jeong H, et al. The impacts of metabolic syndrome and lifestyle on the prevalence of benign prostatic hyperplasia requiring treatment: historical cohort study of 130 454 men. BJU Int. 2019; 123:140–8.

12. Park J, Suh B, Shin DW, Hong JH, Ahn H. Changing patterns of primary treatment in Korean men with prostate cancer over 10 years: a nationwide population based study. Cancer Res Treat. 2016; 48:899–906.

13. Lee SY, Jung KY, Lee IK, Yi SD, Cho YW, Kim DW, et al. Prevalence of treated epilepsy in Korea based on national health insurance data. J Korean Med Sci. 2012; 27:285–90.

14. Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997; 277:467–71.

15. Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014; 15:e484–92.

16. Van Poppel H, Roobol MJ, Chapple CR, Catto JW, N’Dow J, Sonksen J, et al. Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European Association of Urology position and recommendations for 2021. Eur Urol. 2021; 80:703–11.

17. Rapiti E, Fioretta G, Schaffar R, Neyroud-Caspar I, Verkooijen HM, Schmidlin F, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009; 115:5556–65.

18. Aarts MJ, Koldewijn EL, Poortmans PM, Coebergh JW, Louwman M. The impact of socioeconomic status on prostate cancer treatment and survival in the southern Netherlands. Urology. 2013; 81:593–9.

19. Lee DH, Jung HB, Chung MS, Lee SH, Chung BH. The change of prostate cancer treatment in Korea: 5 year analysis of a single institution. Yonsei Med J. 2013; 54:87–91.

20. Mottet N, van den Bergh RC, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021; 79:243–62.
21. Roehrborn CG, Albertsen P, Stokes ME, Black L, Benedict A. First-year costs of treating prostate cancer: estimates from SEER-Medicare data. Prostate Cancer Prostatic Dis. 2009; 12:355–60.

22. Jemal A, Ward E, Wu X, Martin HJ, McLaughlin CC, Thun MJ. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev. 2005; 14:590–5.

23. Afshar N, English DR, Milne RL. Rural-urban residence and cancer survival in high-income countries: a systematic review. Cancer. 2019; 125:2172–84.

24. Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Serv Res. 2005; 40:135–55.

25. Cary C, Odisho AY, Cooperberg MR. Variation in prostate cancer treatment associated with population density of the county of residence. Prostate Cancer Prostatic Dis. 2016; 19:174–9.

27. Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL. Geographic distribution and survival outcomes for rural ppatients with cancer treated in clinical trials. JAMA Netw Open. 2018; 1:e181235.
Fig. 1
Overall survival in pateints with newly diagnosed prostate cancer: (A) insurance type and (B) residence.
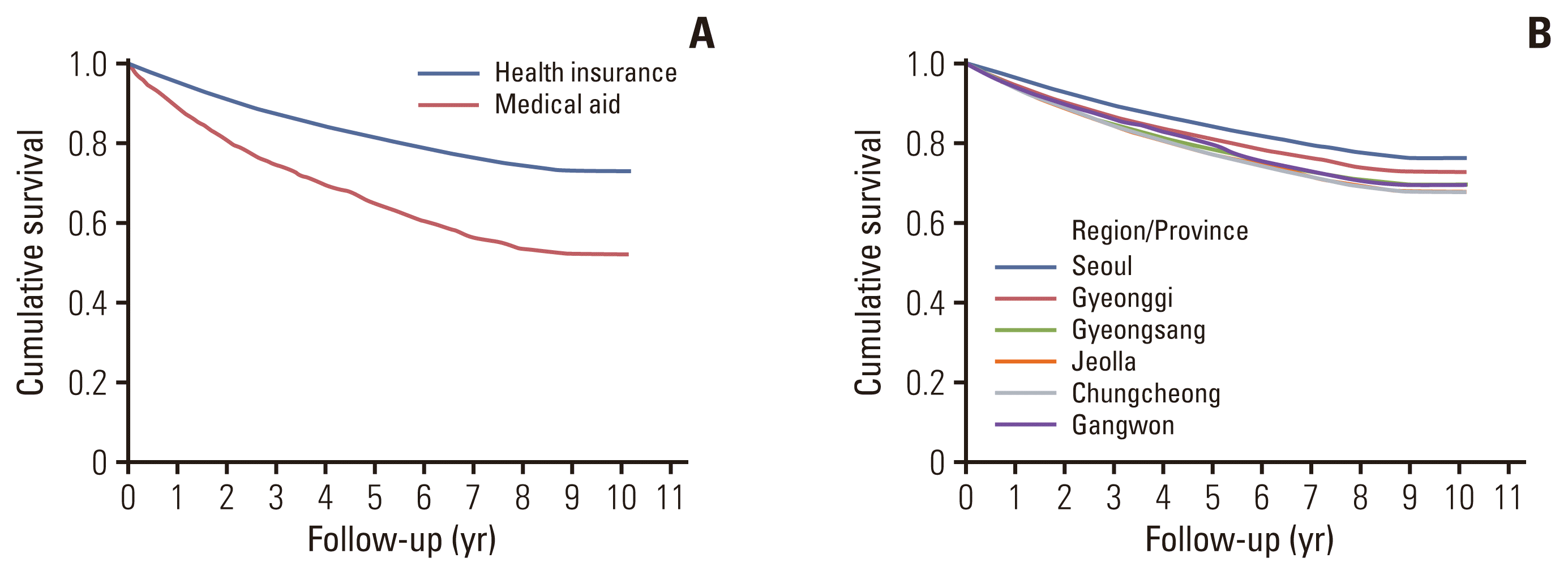
Fig. 2
Proportion of surgical treatment and robotic surgery according to the region. (A) Proportion of surgical treamtent for prostate cancer according to the region. (B) Proportion of robotic surgery for prostate cancer according to the region. OP, operation.
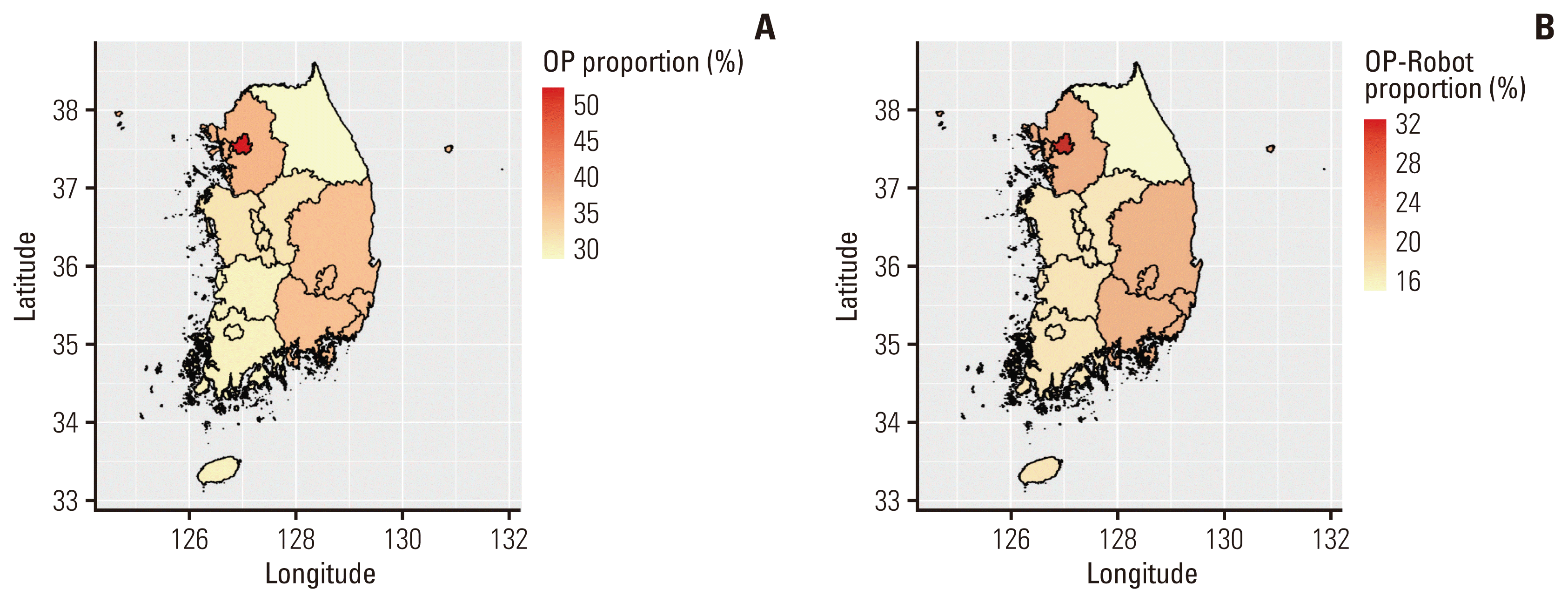
Fig. 3
Oncologic outcomes according to the insurance type and region: (A) androgen-deprivation therapy (ADT)–free survival and (B) overall survival.
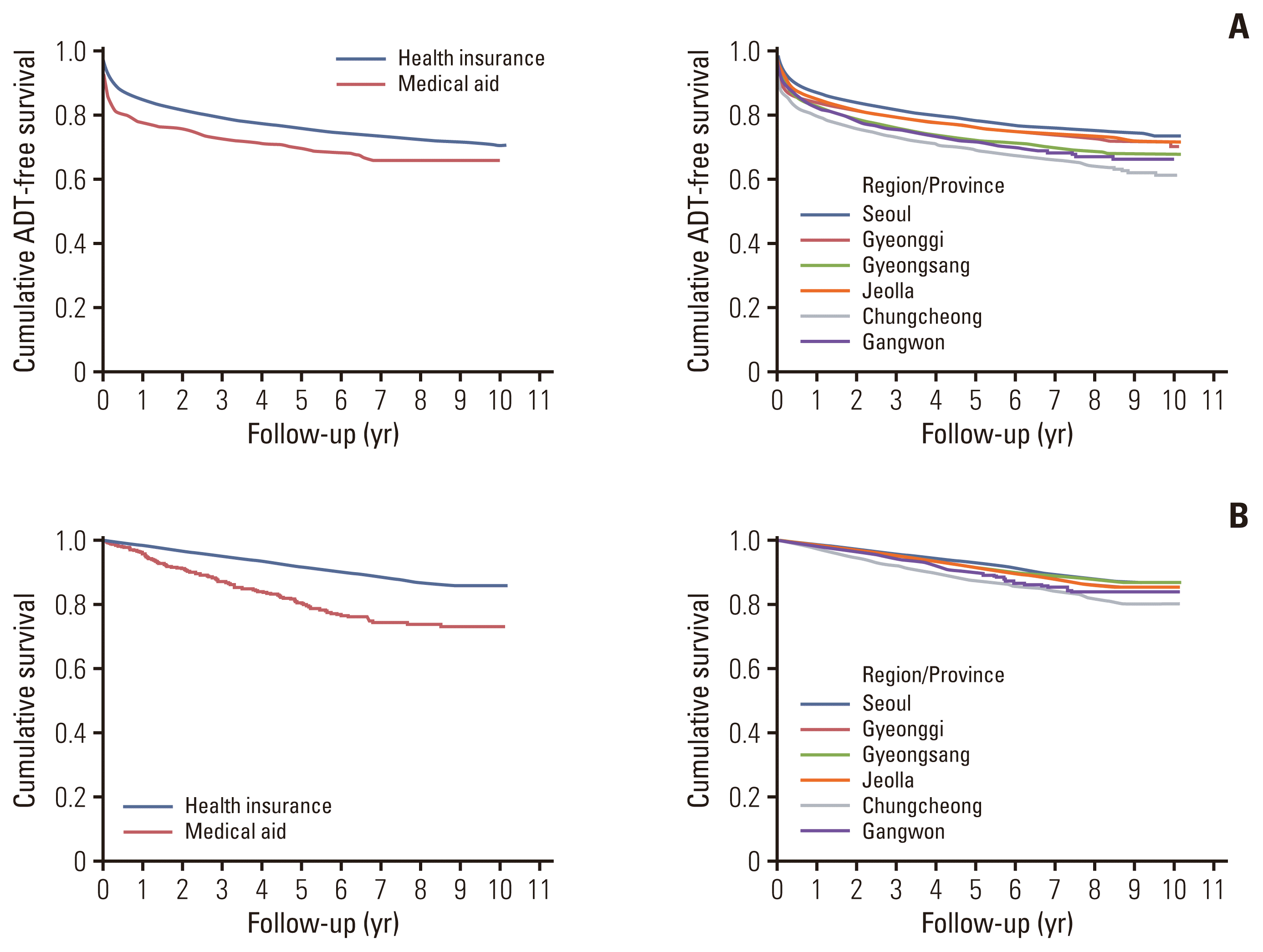
Table 1
Patients characteristics according to insurance type
Table 2
Variables associated with oncological outcomes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download