Abstract
Purpose
Quality assessment of breast cancer treatment in South Korea showed the upward standardization of the grade since 2013, but treatment disparities still have existed. This study analyzed the 5-year trend between 2013 and 2017 in the assessment of breast cancer treatment practice using the Korean health insurance data.
Materials and Methods
All the medical records including surgery, chemotherapy, and radiotherapy for 7,354 patients a year on average were evaluated. Twenty indices consisted of one structural, 17 process-related, and two result-related factors. We calculated the coefficient of variation (CV) annually to determine the variation in adherence rate of evaluation indices according to the type of institution (advanced vs. general hospital vs. clinic).
Results
Based on the initial assessment in 2013, 10 out of 20 indicators showed significant variation among the types of institutions with a CV of less than 0.1%. Six of them had a CV decline of less than 0.1%. The CV was still 0.1% or higher in the four indicators, including the composition of professional staff, the implementation of target therapy, the average length of hospital stay, and the hospitalization cost. Regarding the first grade of assessment, there was a statistically significant relationship between the institution type (p=0.029) and region (metropolitan vs. province, p < 0.001).
In South Korea, the number of breast cancer patients has dramatically increased due to the westernization of life style and the use of hormonal therapy [1]. Between 2006 and 2014, the number of newly diagnosed patients with invasive breast cancer increased by about 60%, according to the National Health Insurance Service and the Korea National Cancer Incidence Database [2,3]. Since the interest in diagnosis and treatment has emerged in South Korea, the Health Insurance Review and Assessment (HIRA) Service conducted annual quality assessment to improve the breast cancer treatment since 2013 [4]. The purpose of the evaluation is to ensure the quality of treatment by minimizing the variation in management according to the type of institution.
Despite years of evaluation efforts, disparities in the breast cancer treatment are still being reported. According to the data released by the Korean Society for Health Equity in 2018, life expectancy in the southeast area outside the metropolitan area in South Korea was 2.4 years shorter than in the metropolitan area, and the number of tertiary general hospitals that can offer the qualified treatment is only half that of the metropolitan area [5]. However, in the actual world, there are still variations in the quality of health care service between regions and types of institutions [6]. Therefore, it is necessary to analyze the improvement trend by detailed indicators and to find out the main factors that are still unresolved and cause the imbalance.
Korean HIRA analyzed the survey based on health insurance medical benefit claims and medical records of the previous year, and compiled the total score of all indicators at each medical institution. The subjects of the survey were female patients aged 18 years or older who underwent surgery for primary breast cancer. Exclusion criteria were as follows: (1) patients with American Joint Committee on Cancer stage 4, (2) bilateral breast cancer, (3) other primary cancerous diseases, diagnosed within 5 years, (4) inflammatory or occult breast cancer, (5) patients who received surgery or treatment in other hospitals, (6) patients who were diagnosed with sarcoma or lymphoma, and (7) pregnant patients. Institutions reporting fewer than 150 breast cancer surgeries were evaluated by census, and those with more than 150 by sampling. Medical records were surveyed annually from 2012 to 2016, and the trends were analyzed based on the data reported the following year.
The evaluation indices comprising a total of 20 indicators were divided into three categories: structure, process, and results. All factors are known to influence the prognosis or to be associated with the treatment decision. A total of 302 indicators from the worldwide quality assessment programs and medical guidelines were compiled into the quality evaluation indicators [7–11]. The National Quality Forum was applied as the criterion for selection, and in order to choose appropriate indices, it was reviewed using the modified Delphi survey, the hospital medical record survey, and the opinion of the experts, which included medical oncologists, breast surgeons, pathologists, and radiation oncologists [12,13].
As a structural component, the composition of professional personnel was investigated. The process category included six diagnostic or reporting-related indicators as follows: breast cancer family history taking, record of patient’s performance status, explanation and permission taking of adjuvant therapy from patient, detailed chemotherapy-related report, radiotherapy dose and field charts, and cancer stage and hormone status related reports by board-certified physicians. Three indicators based on surgery were the faithfulness of surgical pathology report, fulfilling of sentinel or regional lymph node dissection, and clearly negative resected surgical margin rate. Six indicators of systemic adjuvant therapy were commencing adjuvant therapy within 8 weeks after surgery, prescription of adjuvant endocrine therapy in hormone receptor-positive patient and adjuvant chemotherapy recommended by the National Comprehensive Cancer Network (NCCN) guidelines, proportion of patients receiving prophylactic antiemetics in chemotherapy, prescription of target therapy in human epidermal growth factor receptor 2 (HER2)–positive patient, and fulfilling bone mineral density (BMD) before administration of aromatase inhibitors (AI). Two indicators of radiation therapy were the time to start radiotherapy within 6 weeks after surgery or adjuvant chemotherapy, and the proportion of radiotherapy in high-risk patient after mastectomy. The result-related indicators were consisted of the average number of hospitalization days and the average hospitalization cost. The detailed formulae are presented in Fig. 1.
The coefficient of variation (CV) was calculated, and the variation among types of medical institution in the first 2013 survey was evaluated. The CV was defined as the standard deviation of the adherence rate divided by the mean value, and the greater the value, the larger the variation among institution types [14]. A CV ≥ 0.1% reflected large variation among institutional types. The Jonckheere test was conducted to analyze the CV trend every year to evaluate the trends in improvement [15].
Each indicator was summarized and graded into five categories (the first to fifth grade). We analyzed factors affecting the first grade, and the factors used in analyses were the evaluation year, the region to which the institution belongs, and the type of institution. Independent-group t test was used for a comparison between grades and factors. A p-value less than 0.05 was considered statistically significant. Approval of an Institutional Review Board and informed consent was waived because the anonymized patient data were collected from administrative data of HIRA which were open to the public.
The number of survey subjects increased from 4,574 in 2013 to 8,624 in 2017, and the number of institutions also increased from 160 in 2013 to 193 in 2017. The subjects evaluated each year are listed in Table 1. The number of people aged 50 and over has been increased across the board, and the relative increment is particularly notable in the elderly over 80 years of age. The increase in the number of cases undergoing breast-conserving surgery was higher than that of total mastectomy.
The CVs of evaluation indices were extracted and compared to analyze the variations among institutional types that were investigated during the first survey in 2013. Variations were found among with CV value of 0.1% or more in eight structural and process-related indicators (Table 2). Among them, indicators with a relatively larger value of CV (> 0.5%) were the composition of professional personnel, the rates of explanation and permission taking of adjuvant therapy from patient, and the prescription of target therapy in HER2-positive patient. The composition of professional personnel showed the largest CV of 0.631%. In the result categories, the CVs for average hospitalized days and costs were 0.528% and 0.292%, respectively, indicating a large variation among institution types.
Each indicator’s changes were evaluated annually, and Fig. 2 shows the annual trends for each indicator. Among ten indicators with high CVs, the proportion of patients receiving antiemetic drugs, the rate of breast cancer family history taking, record of patient’s performance status, explanation and permission taking of adjuvant therapy from patient, prescription of adjuvant endocrine therapy in hormone receptor-positive patient, and fulfilling BMD before AI administration were six indicators with decreases in CVs of less than 0.1%, indicating that institutional type differences were reduced. However, the four indicators (professional personnel composition, target therapy adherence rate, average number of hospital days, and average hospitalization cost) exhibited CVs with high values of 0.1% or more, showing that the variations were not lowered.
The Jonckheere test was used to assess the 5-year trends of each indicator, and the findings are shown in Table 3. The adherence rate of adjuvant endocrine therapy in hormone receptor-positive patient, prescription of adjuvant chemotherapy recommended by NCCN guidelines, prophylactic antiemetic drug prescriptions in chemotherapy, and fulfilling BMD before AI were indicators that were statistically significant for CV reduction.
The adherence rates of each evaluation index were summarized and graded by institution. Fig. 3 shows the distribution by grade according to year, region, and type of institution. The average ratio of the first grade was 78.6%, the second grade 9.3%, the third grade 7.5%, the fourth grade 1.9%, and the fifth grade 2.7%. The proportion of the first grade increased in 2017 (81.5%) compared with 2013 (72.7%). In the metropolitan area, the ratio of the first grade was higher than that of the provinces (81.8% vs. 74.6%), and the advanced general hospitals presented a markedly higher ratio of the first grade compared with general hospitals (99.4% vs. 76.5%). Factors statistically related to the proportion of the first grade were institution type and region (Table 4). According to the evaluation year, no significant difference was observed.
The purpose of the present study was to evaluate the quality assessment of breast cancer treatment by HIRA in Korea according to indicators and institutional types, and to analyze the factors that require further improvement. This study revealed significant differences in some systemic treatment–related indicators (such as target therapy). Adjuvant treatment after breast cancer surgery depends on the hormone receptor and HER2 type, and various studies are currently in progress on systemic adjuvant therapy [16,17]. The different treatment approaches vary on the physicians’ experience, the institutions, or a multidisciplinary principle, which would have contributed to the high CV [18,19].
According to the annual trend analyses, there was also no statistically significant improvement in structural indicators. In East Asia including South Korea, there have been still structural problems. The fundamental budget for radiation therapy instruments is very high and the number of radiation oncologists are less than surgeons and medical oncologists. The one of reasons for high CV in structure is that the most of specialized doctors including radiation oncologists are affiliated with advanced general hospitals. Interestingly, it was found that there was also no improvement of CV in the systemic chemotherapy field rather than the radiation therapy. The diversity and inconsistency of treatment according to hormone or HER2 status shows that not only in East Asia but also in Europe, recommendations are still being updated [20]. It indicates the need for not only quality assessment but also the development of consensus among multidisciplinary experts.
Similar patterns were seen in studies about breast cancer quality assessment conducted in other East Asian countries [21–23]. In Taiwan, the breast cancer quality evaluation tool consisting of 10 indicators has been implemented since 2007, and during the 5-year evaluation period, high-volume hospitals reported higher adherence rates for pathologic confirmation before surgery and sentinel node sampling in stages 1 and 2 [24]. In Japan, the variation among facilities was analyzed based on seven indicators in 2005; however, no longitudinal studies were conducted [25]. In the present study, Korea showed a high degree of consistency in the area of surgical oncology compared to other East Asian countries. This is considered the result of careful surgical quality control, and it will be used as an international assessment framework.
In the quality evaluation analysis of colorectal cancer conducted by HIRA in Korea, there were also differences in structural elements among the institutional types [26]. During the 6-year evaluation period, there was no significant improvement in the preoperative work-up or postoperative radiotherapy if indicated. The quality evaluations of major carcinomas in Korea, which were conducted since 2011, have continuously been revised and reviewed through additional analysis like previous studies. The present study of breast cancer quality evaluation is also expected to be used as a tool to improve evaluation system.
Because only patients who have had breast cancer surgically resected were eligible for quality assessment of breast cancer treatment by Korean HIRA, this assessment had limited ability to represent the quality of comprehensive breast cancer treatment. Since the initial assessment, there has been less diversity between institutions in the surgical field, which has served to the significant variation in systemic treatments. Additionally, the significance of preventive cancer management emerged as the number of elderly patients increased; nevertheless, this evaluation system did not reflect preventive and generic medicine [27,28].
In conclusion, there was a difference in grades of the treatment quality assessment in breast cancer by region or type of institution, rather than an improvement in grades by year. It is warranted to use the analytical tool of this study on subgroup components rather than just enhancing grades by summarization to validate its applicability as an international evaluation framework.
Notes
Ethical Statement
Approval of an Institute Review Board was waived because the patient data were collected from administrative data without identifiable personal information.
Acknowledgments
This study is based on quality assessment research data of Korean Health Insurance Review & Assessment Service. The authors wish to acknowledge the financial support of the St.Vincent’s hospital, research institute of medical science (SVHR-2022-04).
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.

2. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.

3. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, et al. Nationwide analysis of treatment patterns for Korean breast cancer survivors using National Health Insurance Service data. J Korean Med Sci. 2018; 33:e276.

4. Korean Health Insurance Review and Assessment (HIRA). Evaluation report [Internet]. Wonju: Korean Health Insurance Review and Assessment;2020. [cited 2022 Nov 27]. Available from: https://www.hira.or.kr/ra/eval/asmWrptPopup.do?evlCd=19&pgmid=HIRAA030004000000
.
5. Korean Society for Health Equity in Health [Internet]. Seoul: Korean Society for Health Equity in Health;2022. [cited 2022 Nov 27]. Available from: http://healthequity.or.kr/
.
6. Kim SH, Kang S, Song MK. Intensity of care at the end of life among older adults in Korea. J Palliat Care. 2018; 33:47–52.

7. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009; 20:1319–29.

8. Han D, Hogeveen S, Sweet Goldstein M, George R, Brezden-Masley C, Hoch J, et al. Is knowledge translation adequate? A quality assurance study of staging investigations in early stage breast cancer patients. Breast Cancer Res Treat. 2012; 132:1–7.

9. Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition: summary document. Oncol Clin Pract. 2008; 4:74–86.
10. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009; 99:488–90.

11. Biganzoli L, Marotti L, Hart CD, Cataliotti L, Cutuli B, Kuhn T, et al. Quality indicators in breast cancer care: an update from the EUSOMA working group. Eur J Cancer. 2017; 86:59–81.

12. National Quality Forum. National voluntary consensus standards for quality of cancer care. Washington, DC: National Quality Forum;2009.
13. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corp;2001.
14. Bedeian AG, Mossholder KW. On the use of the coefficient of variation as a measure of diversity. Org Res Methods. 2000; 3:285–97.

15. Lunneborg CE. Jonckheere–Terpstra test. Everitt B, Howell D, editors. Encyclopedia of statistics in behavioral science. Hoboken, NJ: John Wiley & Sons;2005.

16. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline update on ovarian suppression. J Clin Oncol. 2016; 34:1689–701.

17. Schlam I, Tarantino P, Morganti S, Lynce F, Trapani D, Mayer EL, et al. Emerging targeted therapies for early breast cancer. Drugs. 2022; 82:1437–51.

18. Gilligan MA, Neuner J, Zhang X, Sparapani R, Laud PW, Nattinger AB. Relationship between number of breast cancer operations performed and 5-year survival after treatment for early-stage breast cancer. Am J Public Health. 2007; 97:539–44.

19. Guller U, Safford S, Pietrobon R, Heberer M, Oertli D, Jain NB. High hospital volume is associated with better outcomes for breast cancer surgery: analysis of 233,247 patients. World J Surg. 2005; 29:994–9.

20. Giorgi Rossi P, Lebeau A, Canelo-Aybar C, Saz-Parkinson Z, Quinn C, Langendam M, et al. Recommendations from the European Commission Initiative on Breast Cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, HER-2 negative. Br J Cancer. 2021; 124:1503–12.

21. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014; 15:e279–89.

22. Kurebayashi J, Miyoshi Y, Ishikawa T, Saji S, Sugie T, Suzuki T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: Based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer. 2015; 22:235–44.

23. Lin CH, Liau JY, Lu YS, Huang CS, Lee WC, Kuo KT, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarkers Prev. 2009; 18:1807–14.

24. Ou-Yang F, Hsu NC, Juan CH, Huang HI, Moi SH, Chen FM, et al. Breast cancer quality of care in Taiwan in relation to hospital volume: a population-based cohort study. Asia Pac J Clin Oncol. 2015; 11:308–13.

25. Mukai H, Higashi T, Sasaki M, Sobue T. Quality evaluation of medical care for breast cancer in Japan. Int J Qual Health Care. 2016; 28:110–3.

26. Choi KH, Song JH, Jang HS, Kim SH, Lee JH. Current trends in the quality assessment of colorectal cancer practice and treatment in South Korea during 2012–2017. Cancer Res Treat. 2021; 53:487–96.

Fig. 1
Definition of 20 evaluation indices. AI, aromatase inhibitors; DRG, diagnosis-related group; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factore receptor 2; NCCN, National Comprehensive Cancer Network; SISH, silver-enhanced in situ hybridization.
Fig. 2
Coefficient of variation by institution type from 2013 to 2017. (A) Structural indicator and report fidelity. (B) Surgery-related indicators. (C) Systemic therapy–related indicators. (D) Radiotherapy-related indicators. (E) Result indicators. AI, aromatase inhibitors.
Fig. 3
Distribution of grades according to evaluation year in 2013 vs. 2017 (A), metropolitan area vs. provinces (B), and advanced general hospital vs. general hospital (C).
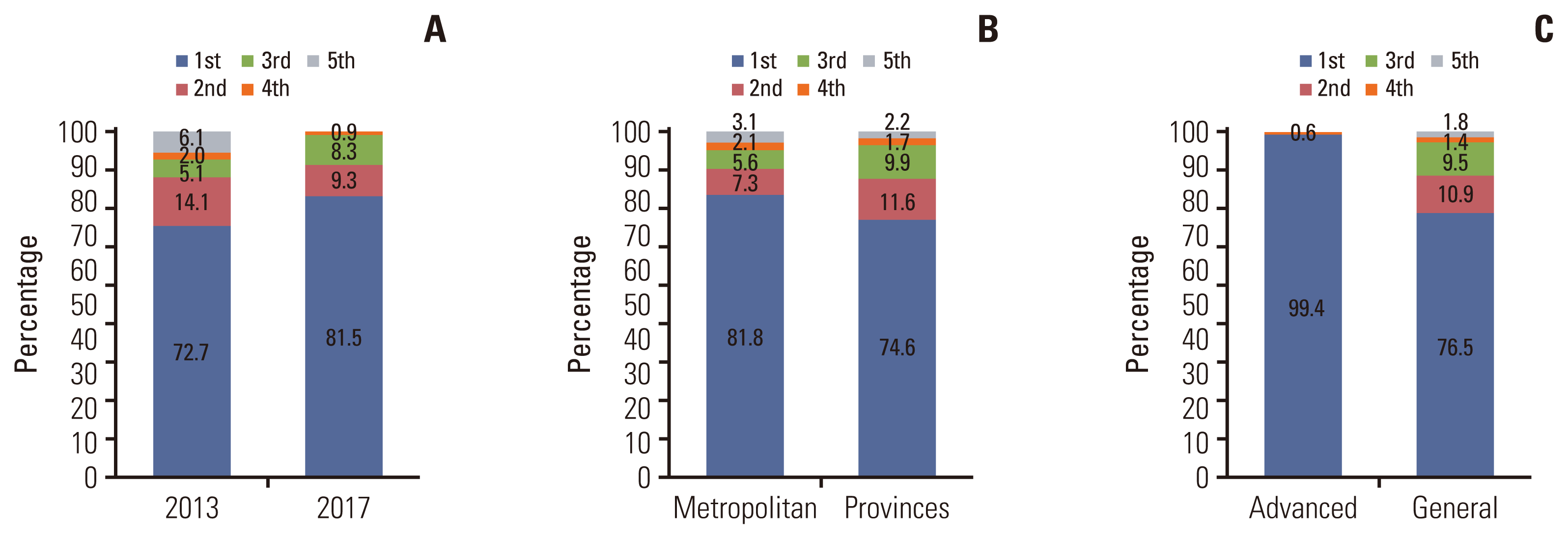
Table 1
Study population
| Characteristic | Cases per year | |||||
|---|---|---|---|---|---|---|
| 2017 | 2016 | 2015 | 2014 | 2013 | Average | |
| No. of institutions | ||||||
| Advanced general hospital | 43 | 43 | 43 | 43 | 44 | 43.2 |
| General hospital | 118 | 114 | 113 | 116 | 95 | 111.2 |
| Hospital | 21 | 22 | 19 | 18 | 15 | 19.0 |
| Clinic | 11 | 8 | 10 | 8 | 6 | 8.6 |
| Age (yr) | ||||||
| 18–29 | 68 | 46 | 57 | 64 | - | 58.8 |
| 30–39 | 703 | 668 | 714 | 739 | - | 706.0 |
| 40–49 | 2,784 | 2,765 | 2,677 | 2,676 | - | 2,725.5 |
| 50–59 | 2,710 | 2,510 | 2,396 | 2,404 | - | 2,505.0 |
| 60–69 | 1,449 | 1,325 | 1,192 | 1,111 | - | 1,269.3 |
| 70–79 | 720 | 640 | 639 | 621 | - | 655.0 |
| ≥ 80 | 190 | 124 | 107 | 95 | - | 129.0 |
| Surgery type | ||||||
| Breast-conserving surgery | 5,661 | 5,323 | 5,052 | 4,967 | 2,976 | 4,795.8 |
| Mastectomy | 2,963 | 2,755 | 2,730 | 2,743 | 1,598 | 2,557.8 |
| Pathologic stagea) | ||||||
| I | 3,882 | 3,649 | - | 3,557 | 2,110 | 3,299.5 |
| II | 3,488 | 3,254 | - | 3,031 | 1,843 | 2,904.0 |
| III | 1,254 | 1,174 | - | 1,122 | 621 | 1,042.8 |
Table 2
Coefficient variation among types of institutions at initial evaluation
Table 3
Jonckheere test for analyzing trend of variation coefficient for 5 years




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download