Introduction
Materials and Methods
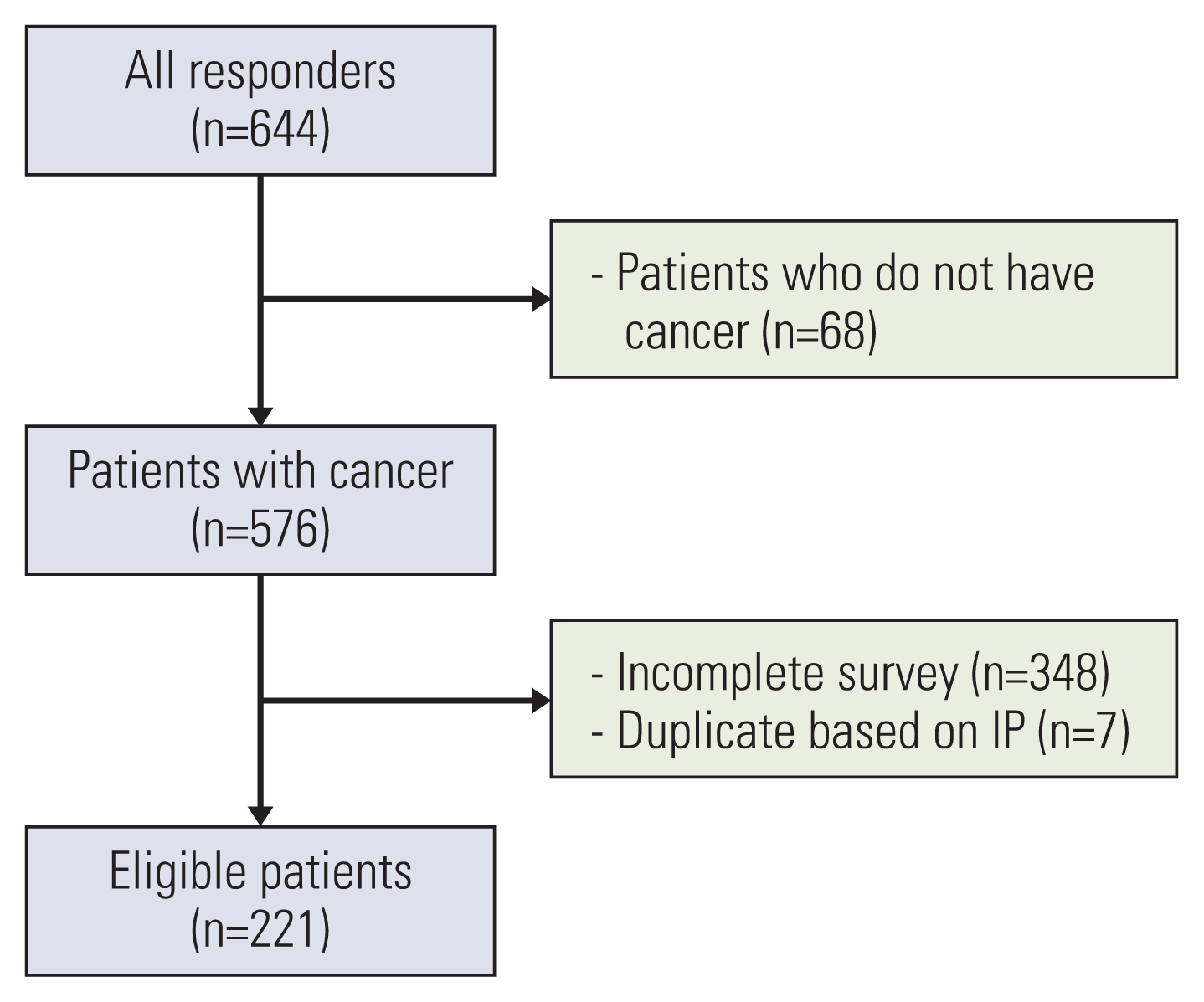
1. Study population
2. Questionnaire and measurement
3. Statistical analysis
Results
1. Baseline characteristics
Table 1
| Total (n=221) | CAM usage | p-valuea) | Anthelmintics | p-valuea) | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Non-user (n=48) | User (n=173) | Non-userb) (n=145) | Userb) (n=28) | ||||
| Age (yr) | 52 (45–61) | 57 (45–65.75) | 52 (44.5–60) | 0.028 | 52 (45–60) | 50 (41.5–58) | 0.348 |
|
|
|||||||
| Sex | |||||||
|
|
|||||||
| Male | 114 (51.6) | 33 (68.8) | 81 (46.8) | 0.007 | 68 (46.9) | 13 (46.4) | 0.964 |
|
|
|||||||
| Female | 107 (48.4) | 15 (31.2) | 92 (53.2) | 77 (53.1) | 15 (53.6) | ||
|
|
|||||||
| Region | |||||||
|
|
|||||||
| Seoul, metropolitan areas | 99 (44.8) | 21 (43.8) | 78 (45.1) | 0.869 | 70 (48.3) | 8 (28.6) | 0.055 |
|
|
|||||||
| Others | 122 (55.2) | 27 (56.3) | 95 (54.9) | 75 (51.7) | 20 (71.4) | ||
|
|
|||||||
| Education | |||||||
|
|
|||||||
| College graduate or higher | 78 (35.3) | 17 (35.4) | 61 (35.3) | 0.984 | 52 (35.9) | 9 (32.1) | 0.706 |
|
|
|||||||
| Less than college graduate | 143 (64.7) | 31 (64.6) | 112 (64.7) | 93 (64.1) | 19 (67.9) | ||
|
|
|||||||
| Health insurance | |||||||
|
|
|||||||
| National Health Insurance | 212 (95.9) | 47 (97.9) | 165 (95.4) | 0.381 | 140 (96.6) | 25 (89.3) | 0.121 |
|
|
|||||||
| Medical aid, type 1 and 2 | 9 (4.1) | 1 (2.1) | 8 (4.6) | 5 (3.4) | 3 (10.7) | ||
|
|
|||||||
| Diagnosis | |||||||
|
|
|||||||
| Kidney cancer | 78 (35.3) | 17 (35.4) | 61 (35.3) | 0.191 | 56 (38.6) | 5 (17.9) | 0.002 |
|
|
|||||||
| Breast cancer | 40 (18.1) | 5 (10.4) | 35 (20.2) | 29 (20.0) | 6 (21.4) | ||
|
|
|||||||
| Esophageal cancer | 39 (17.6) | 14 (29.2) | 25 (14.5) | 24 (16.6) | 1 (3.6) | ||
|
|
|||||||
| Other malignancies | 24 (10.9) | 4 (8.3) | 20 (11.6) | 17 (11.7) | 3 (10.7) | ||
|
|
|||||||
| Neuroendocrine tumor | 23 (10.4) | 6 (12.5) | 17 (9.8) | 9 (6.2) | 8 (28.6) | ||
|
|
|||||||
| Colorectal cancer | 10 (4.5) | 2 (4.2) | 8 (4.6) | 5 (3.4) | 3 (10.7) | ||
|
|
|||||||
| Hepatobiliary pancreatic cancer | 7 (3.2) | 0 | 7 (4.0) | 5 (3.4) | 2 (7.1) | ||
|
|
|||||||
| Metastasis | |||||||
|
|
|||||||
| Yes | 103 (46.6) | 27 (56.3) | 76 (43.9) | 0.130 | 55 (37.9) | 21 (75.0) | < 0.001 |
|
|
|||||||
| No | 118 (53.4) | 21 (43.8) | 97 (56.1) | 90 (62.1) | 7 (25.0) | ||
|
|
|||||||
| Duration of disease (mo) | 20 (7–46.5) | 8.5 (5–51.25) | 23 (9–46) | 0.080 | 19 (7.5–43) | 33 (23–85) | 0.002 |
|
|
|||||||
| Anti-cancer treatment (multiple) | |||||||
|
|
|||||||
| Surgery | 163 (73.8) | 34 (70.8) | 129 (74.6) | 110 (75.9) | 19 (67.9) | ||
|
|
|||||||
| Chemotherapy | 113 (51.1) | 25 (52.1) | 88 (50.9) | 72 (49.7) | 16 (57.1) | ||
|
|
|||||||
| Radiation therapy | 70 (31.7) | 12 (25.0) | 58 (33.5) | 46 (31.7) | 12 (42.9) | ||
|
|
|||||||
| Concurrent chemoradiation therapy | 41 (18.6) | 11 (22.9) | 30 (17.3) | 25 (17.2) | 5 (17.9) | ||
|
|
|||||||
| Hormone therapy | 21 (9.5) | 3 (6.3) | 18 (10.4) | 14 (9.7) | 4 (14.3) | ||
|
|
|||||||
| Palliative therapy | 9 (4.1) | 1 (2.1) | 5 (2.9) | 4 (2.8) | 1 (3.6) | ||
|
|
|||||||
| Et cetera | 22 (10.0) | 3 (6.3) | 19 (11.0) | 10 (6.9) | 9 (32.1) | ||
|
|
|||||||
| ECOG PS | |||||||
|
|
|||||||
| 0 | 103 (46.6) | 21 (43.8) | 82 (47.4) | 0.654 | 67 (46.2) | 15 (53.6) | 0.475 |
|
|
|||||||
| 1–4 | 118 (53.4) | 27 (56.3) | 91 (52.6) | 78 (53.8) | 13 (46.4) | ||
|
|
|||||||
| Private insurance | |||||||
|
|
|||||||
| No | 40 (18.1) | 12 (25.0) | 28 (16.2) | 0.160 | 23 (15.9) | 5 (17.9) | 0.795 |
|
|
|||||||
| Yes | 181 (81.9) | 36 (75.0) | 145 (83.8) | 122 (84.1) | 23 (82.1) | ||
|
|
|||||||
| Covering medical expenses with private insurance | |||||||
|
|
|||||||
| No | 70 (31.7) | 17 (35.4) | 53 (30.6) | 0.529 | 41 (28.3) | 12 (42.9) | 0.125 |
|
|
|||||||
| Yes (90% or more of expenses) | 151 (68.3) | 31 (64.6) | 120 (69.4) | 104 (71.7) | 16 (57.1) | ||
|
|
|||||||
| Family income (per month) (won) | |||||||
|
|
|||||||
| < 3,000,000 | 88 (39.8) | 18 (37.5) | 70 (40.5) | 0.701 | 56 (38.6) | 14 (50.0) | 0.027 |
|
|
|||||||
| ≥ 3,000,000 and < 7,000,000 | 100 (45.2) | 21 (43.8) | 79 (45.7) | 72 (49.7) | 7 (25.0) | ||
|
|
|||||||
| ≥ 7,000,000 | 33 (14.9) | 9 (18.8) | 24 (13.9) | 17 (11.7) | 7 (25.0) | ||
|
|
|||||||
| Expenses for cancer treatment (per year) (won) | |||||||
|
|
|||||||
| < 10,000,000 | 116 (52.5) | 28 (58.3) | 88 (50.9) | 0.359 | 76 (52.4) | 12 (42.9) | 0.354 |
|
|
|||||||
| ≥ 10,000,000 | 105 (47.5) | 20 (41.7) | 85 (49.1) | 69 (47.6) | 16 (57.1) | ||
|
|
|||||||
| Have you ever heard of CAM? | |||||||
|
|
|||||||
| Yes | 151 (68.3) | 30 (62.5) | 121 (69.9) | 0.327 | 95 (65.5) | 26 (92.9) | 0.004 |
|
|
|||||||
| No | 70 (31.7) | 18 (37.5) | 52 (30.1) | 50 (34.5) | 2 (7.1) | ||
|
|
|||||||
| Do you believe CAM’s efficacy and safety? | |||||||
|
|
|||||||
| No | 87 (39.4) | 25 (52.1) | 62 (35.8) | 0.042 | 52 (35.9) | 10 (35.7) | 0.988 |
|
|
|||||||
| Yes | 134 (60.6) | 23 (47.9) | 111 (64.2) | 93 (64.1) | 18 (64.3) | ||
|
|
|||||||
| Types of experienced CAM | 1 (0–2) | 0 (0–0) | 1 (1–2) | < 0.001 | 1 (1–2) | 2 (1–4) | 0.005 |
Table 2
| Type of CAMa) | CAM experience (n=155)b) | Current user (n=58) |
|---|---|---|
| Alternative medical system | 40 (25.8) | 10 (17.2) |
| Mind-body therapies | 28 (18.1) | 7 (12.1) |
| Biologically based therapies | 136 (87.7) | 54 (93.1) |
| Manipulative and body-based methods | 78 (50.3) | 13 (22.4) |
| Energy therapies | 51 (32.9) | 13 (22.4) |
| Others | 11 (7.1) | 0 |
a) Multiple choices were allowed among the NCCIH category [18],
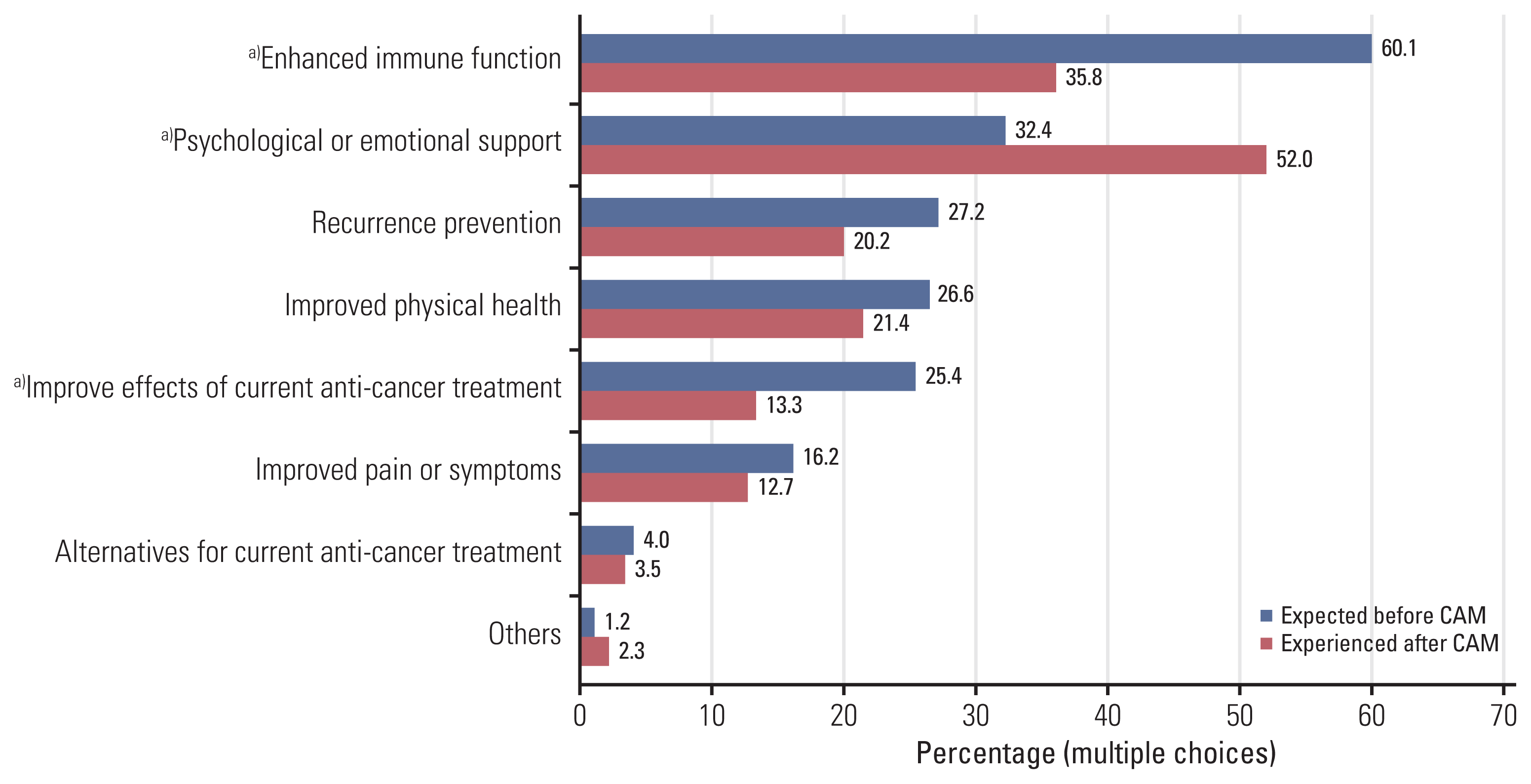
2. Expectations and experiences regarding CAM use
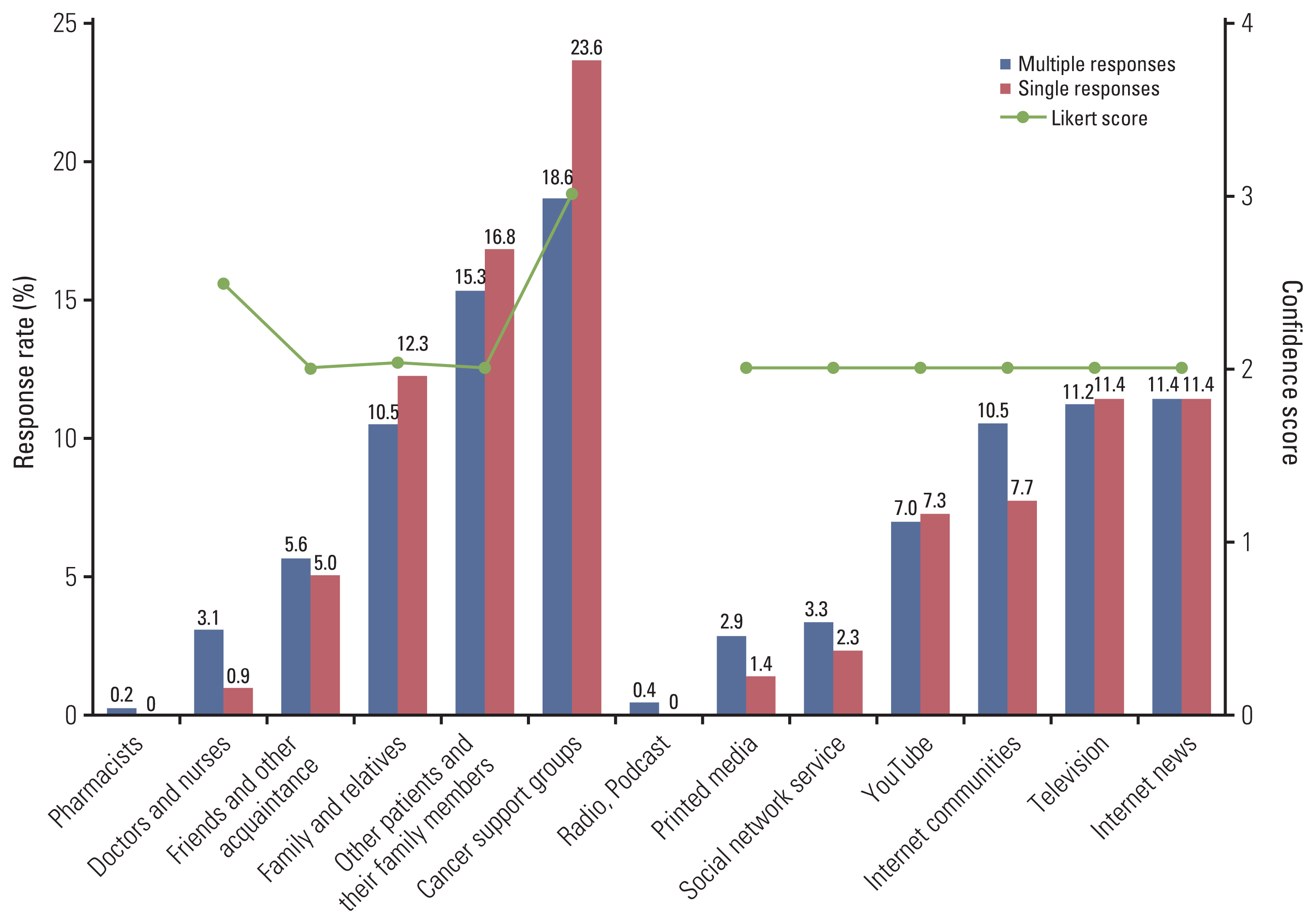
3. Source of information and reliabilities
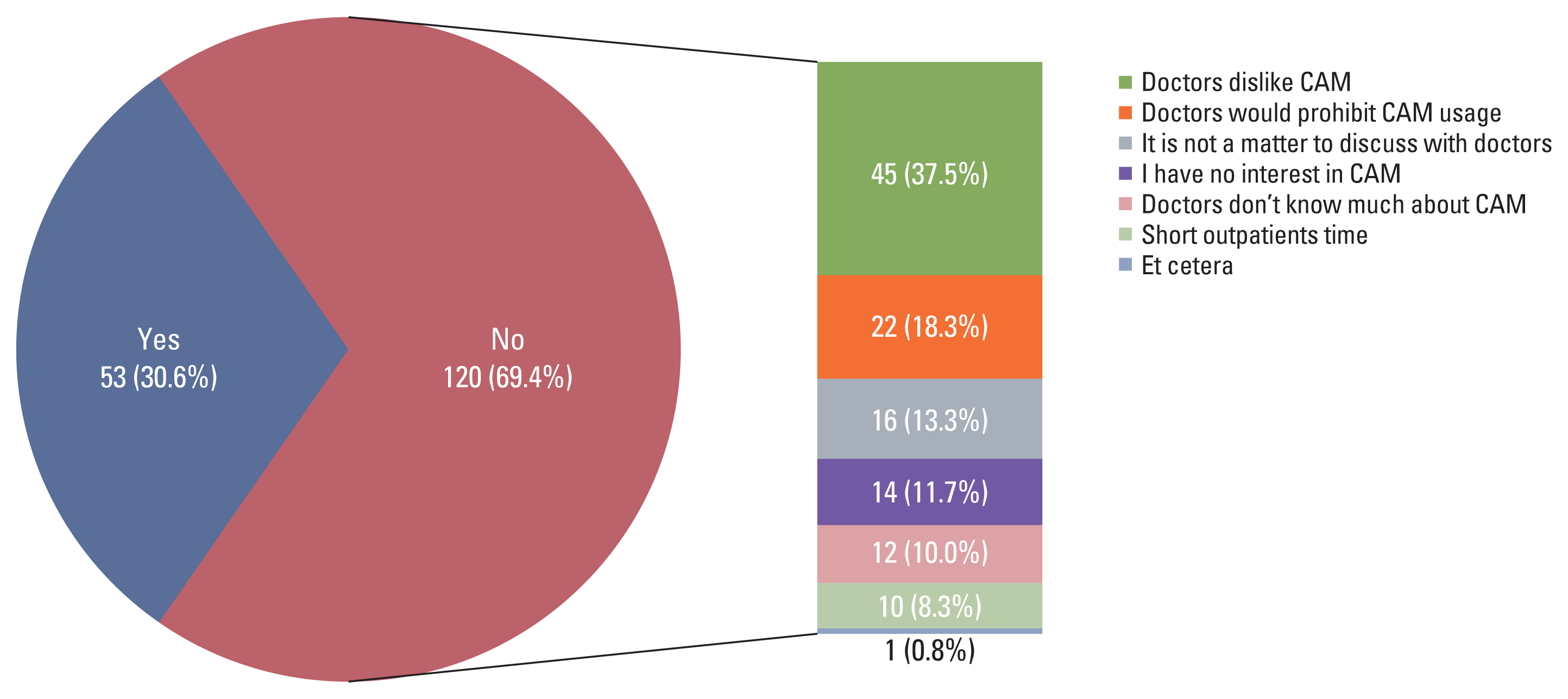
4. Discussion with physicians about CAM usage
5. Use of anthelmintics and associated factors
Table 3
| Variable | OR (95% CI) | |
|---|---|---|
| Univariable | Multivariablea) | |
| Duration of diseaseb) | 1.01 (1.00–1.02) | |
| Ageb) | 0.98 (0.94–1.02) | 0.89 (0.84–0.95) |
| Female sex | 1.02 (0.45–2.29) | |
| Region (non-metropolitan areas) | 2.33 (0.97–5.64) | |
| Education (college graduate or higher) | 1.18 (0.50–2.80) | |
| Insurance (Medical aid, type 1 and 2) | 3.36 (0.76–14.96) | |
| Diagnosis | ||
| Kidney cancer | 1 | |
| Breast cancer | 2.32 (0.65–8.24) | |
| Esophageal cancer | 0.47 (0.05–4.21) | |
| Other malignancies | 1.98 (0.43–9.14) | |
| Neuroendocrine tumor | 9.96 (2.66–37.29) | |
| Colorectal cancer | 6.72 (1.23–36.74) | |
| Hepatobiliary pancreatic cancer | 4.48 (0.69–29.29) | |
| Presence of metastasis (yes) | 4.91 (1.96–12.30) | 10.88 (3.39–34.86) |
| ECOG PS (0) | 1.34 (0.60–3.02) | |
| Private insurance (no) | 1.15 (0.40–3.34) | |
| Family income (per month) (won) | ||
| Under 3,000,000 | 1 | |
| 3,000,000–7,000,000 | 0.39 (0.15–1.03) | |
| 7,000,000 or higher | 1.65 (0.57–4.74) | |
| Expenses for cancer treatment (per year) (≥ 10,000,000 won) | 1.47 (0.65–3.32) | |
| Have you ever heard of CAM? (yes) | 6.84 (1.56–30.01) | 5.57 (1.01–30.72) |
| Do you believe in CAM? (no) | 0.99 (0.43–2.31) | |
| Numbers of CAM types that are usedb) | 1.82 (1.32–2.52) | 1.98 (1.29–3.05) |
| Do you know enough about CAM?c) | 2.03 (1.25–3.29) | |
| Do you have interest in CAM? (yes) | 1.23 (0.79–1.93) | |
| Have you ever discussed with your physicians about CAM? (Yes) | 2.28 (0.995–5.20) | |
| Have you experienced side effects from CAM? (yes) | 4.05 (1.61–10.21) | 5.10 (1.46–17.75) |
CAM, complementary and alternative medicine; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio.
a) Performed using multivariable logistic regression model with stepwise, backward selection for anthelmintics usage. Age, sex, and variables, which showed significance in the univariable analysis were included in the model (i.e., duration of disease, presence of metastasis, have you heard of CAM?, Numbers of CAM types that are used, Do you know enough about CAM?, Have you experienced side effects from CAM?),




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download