Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
This study was approved by the institutional review boards of all participating institutions, and the trial was registered at ClinicalTrials.gov, number NCT02364466. Informed consent was obtained from all patients for being included in the study.
Author Contributions
Conceived and designed the analysis: Yoon DY, Kim WS.
Collected the data: Cho H, Yoon DY, Shin DY, Koh Y, Yoon SS, Kim SJ, Do YR, Lee GW, Kwak JY, Park Y, Kang HJ, Yi JH, Yoo KH, Lee WS, Park BB, Jo JC, Eom HS, Kim HJ, Jeong SH, Won YW, Sohn BS, Kwon JH, Suh C, Kim WS.
Contributed data or analysis tools: Cho H, Shin DY, Koh Y, Yoon SS, Kim SJ, Do YR, Lee GW, Kwak JY, Park Y, Kim MK, Kang HJ, Yi JH, Yoo KH, Lee WS, Park BB, Jo JC, Eom HS, Kim HJ, Jeong SH, Won YW, Sohn BS, Kwon JH, Suh C, Kim WS.
Performed the analysis: Cho H, Yoon DY, Kim WS.
Wrote the paper: Cho H, Yoon DY.
References
Fig. 1
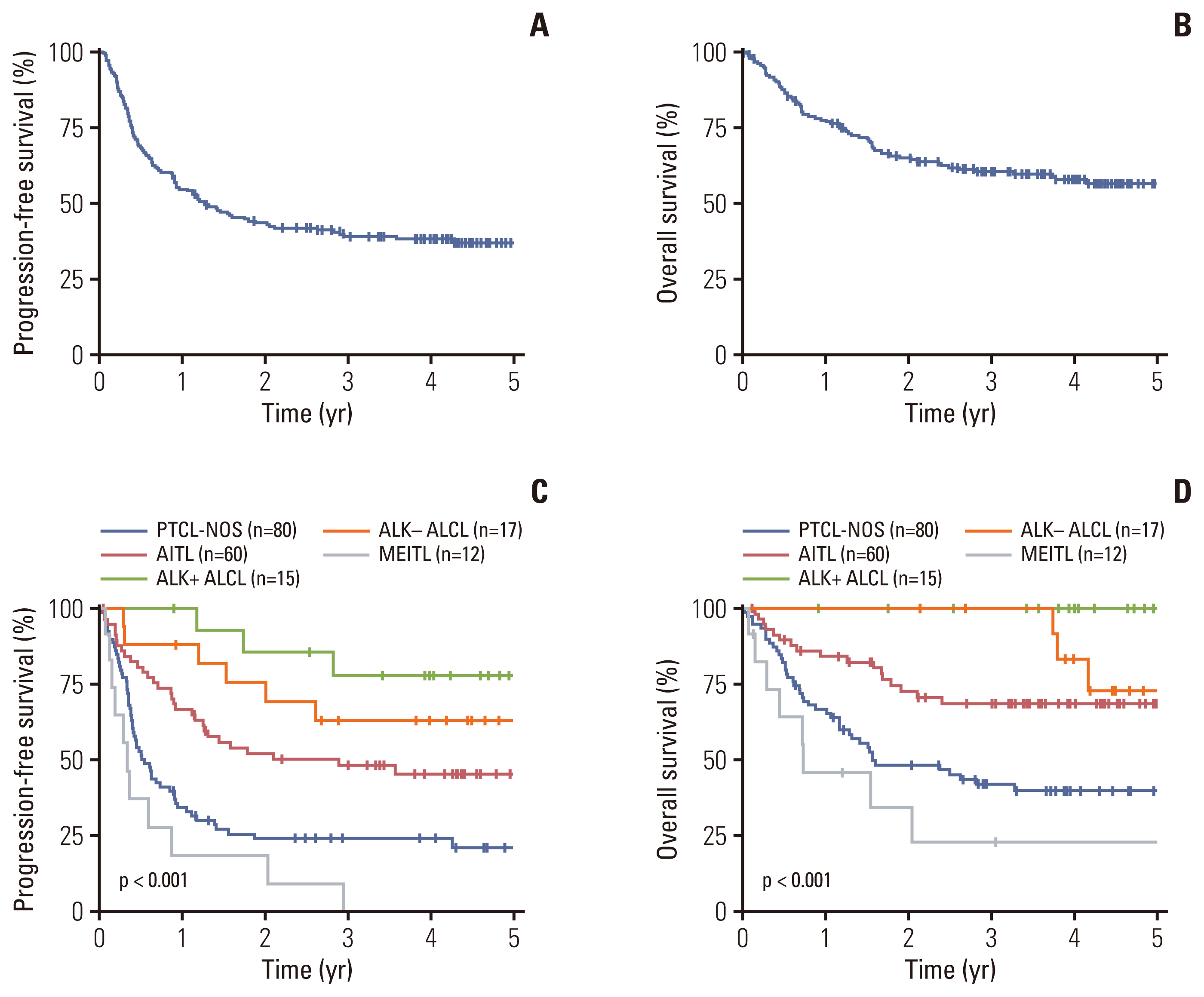
Fig. 2
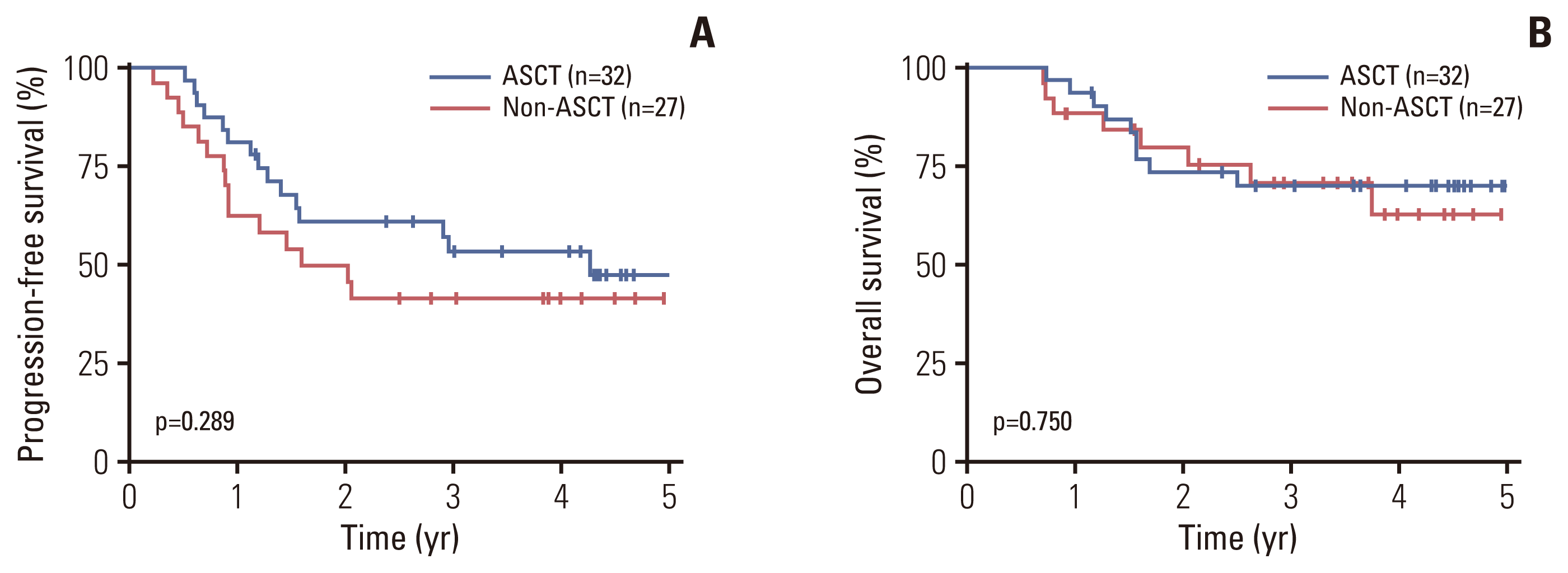
Fig. 3
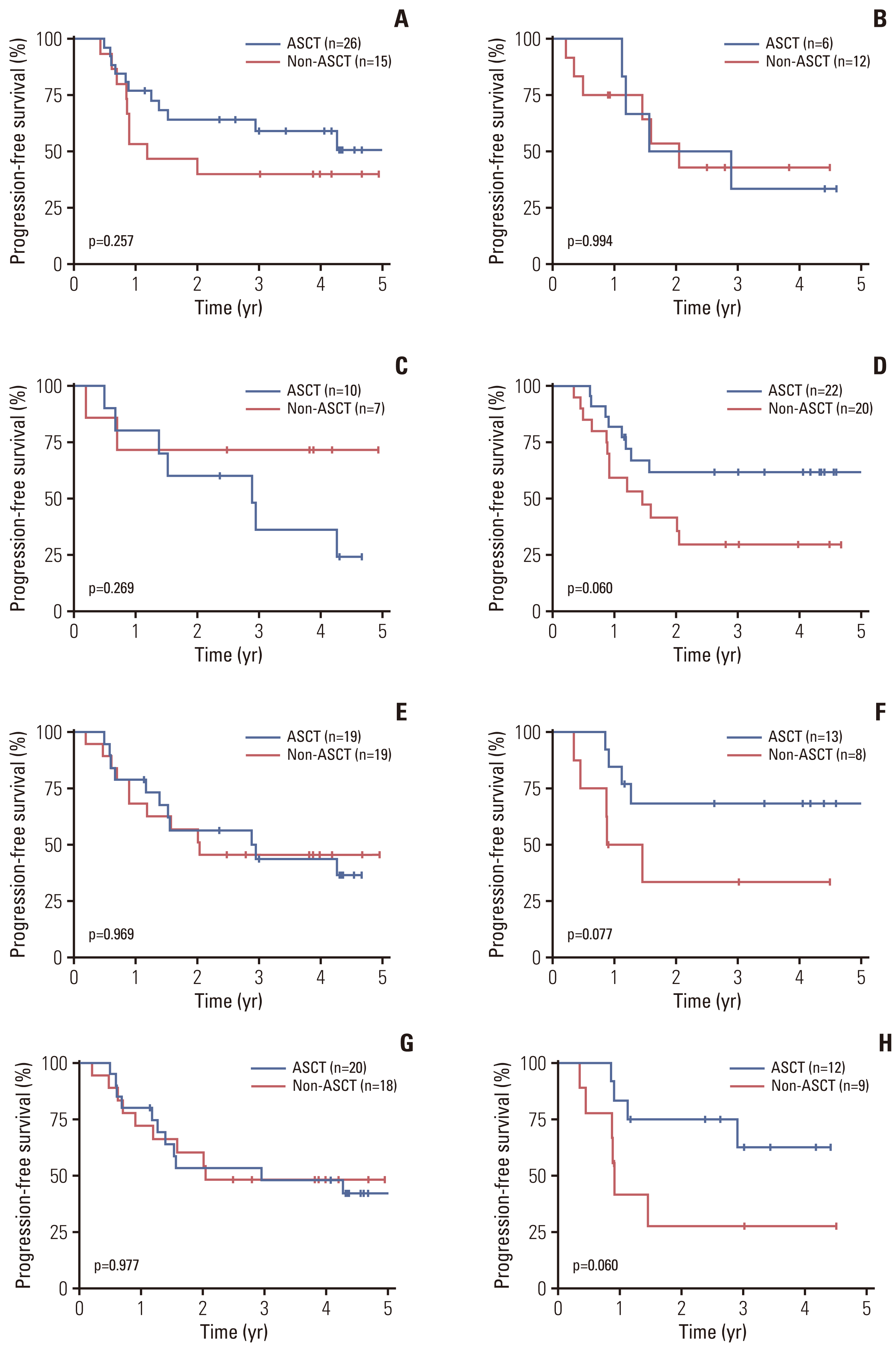
Fig. 4
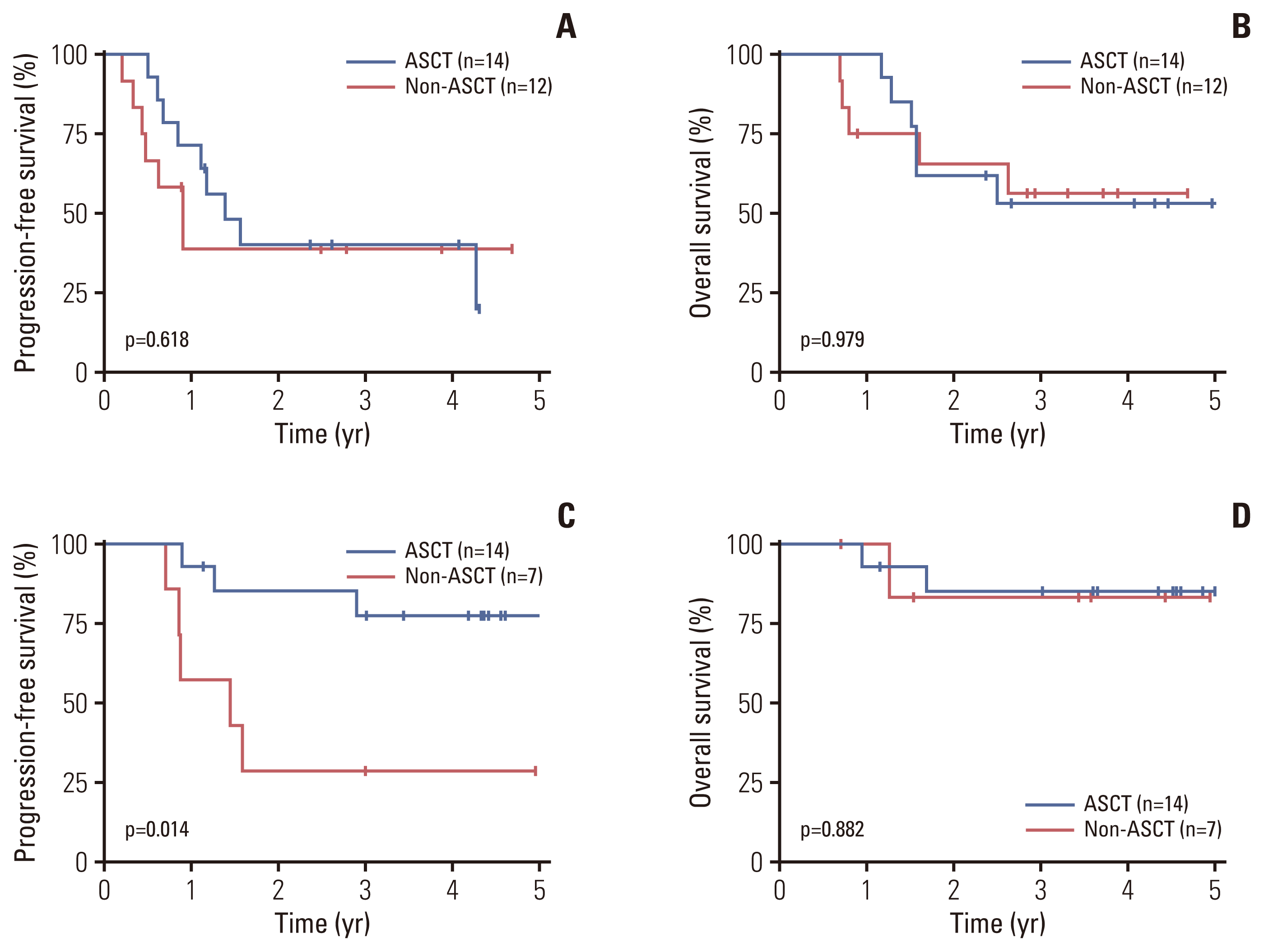
Table 1
Table 2
Values are presented as number (%) unless otherwise indicated. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; ECOG PS, Eastern Cooperative Oncology Group performance status; ICE, ifosfamide, carboplatin, and etoposide; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; PTCL-U, peripheral T-cell lymphoma–unspecified.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download