INTRODUCTION
Gastrointestinal (GI) endoscopes are used worldwide for the screening, diagnosis, and treatment of GI diseases. Although the incidence of pathogen transmission is very low,
1
Salmonella spp.,
Pseudomonas, Mycobacteria,
Helicobacter pylori, HBV, HCV, and other pathogens can be transmitted through GI endoscopy.
23 These microorganisms can be transmitted by the endoscope and its accessories, contaminated reprocessing equipment, and/or contaminated disinfectant solution.
4 Most endoscope-related microbial transmissions can be prevented by adequate endoscope reprocessing. Many organizations have introduced guidelines for the reprocessing of GI endoscopes.
567 For these guidelines to be followed diligently in practice, the reprocessing procedure must be simple, fast, and inexpensive. Currently, many endoscopic units use automated endoscope reprocessors (AERs). Glutaraldehyde solution (2%) is the most commonly used disinfectant in GI endoscope reprocessing,
8 and according to multi-society guidelines, at least 20 minutes of contact time at 20℃ is required for it to be effective.
6 In contrast, a contact time of only 5–12 minutes at 20℃ is required for another disinfectant, ortho-phthalaldehyde (OPA), with the recommended contact time varying according to country.
9 Although use of OPA is more time-efficient than use of glutaraldehyde solution, OPA is more expensive than glutaraldehyde solution.
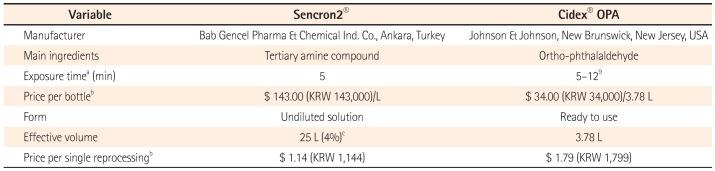
Given this tradeoff, we evaluated the efficacy and safety of a relatively inexpensive tertiary amine compound (TAC) solution for reprocessing endoscopes. This solution requires only 5 minutes of contact time at 20℃ and is already used in European countries (Certificate Number: 1984-MDD-14-262) (
Table 1).
Table 1
Comparison of Sencron2® and Cidex®OPA
|
Variable |
Sencron2® |
Cidex® OPA |
|
Manufacturer |
Bab Gencel Pharma & Chemical Ind. Co., Ankara, Turkey |
Johnson & Johnson, New Brunswick, New Jersey, USA |
|
Main ingredients |
Tertiary amine compound |
Ortho-phthalaldehyde |
|
Exposure timea (min) |
5 |
5–129
|
|
Price per bottleb
|
$ 143.00 (KRW 143,000)/L |
$ 34.00 (KRW 34,000)/3.78 L |
|
Form |
Undiluted solution |
Ready to use |
|
Effective volume |
25 L (4%)c
|
3.78 L |
|
Price per single reprocessingb
|
$ 1.14 (KRW 1,144) |
$ 1.79 (KRW 1,799) |

Go to :

METHODS
This was a prospective, randomized study conducted at a tertiary referral center in Seoul, Korea, between February 2014 and May 2014. The study protocol was approved by the institutional review board of Kangbuk Samsung Hospital, Seoul, Korea.
1. Endoscopes and AERs
We used colonoscopes (CF-H260AI and CF-Q260AI, Olympus Optical Co., Ltd., Tokyo, Japan) and two AERs (CYW-201, Choyang Medical Industry Ltd., Seongnam, Korea), which have the same duration of use. We used only one type of disinfectant for each AER during the study period. Colonoscopes were randomly assigned to each AER at a 1:1 ratio using a computer-generated random code list, without categorization according to the endoscopist. Fifty cases were processed by each AER.
2. Endoscope Reprocessing and Disinfection
Based on the guidelines for cleaning and disinfecting GI endoscopes as reported by the Korean Society of Gastrointestinal Endoscopy in 2012,
10 we reprocessed the endoscopes as follows.
After each use of the colonoscope, pre-cleaning was performed at the point of use. The pre-cleaning procedure included wiping the surfaces and flushing the channels with a detergent solution (Cidezyme®, Johnson & Johnson, New Brunswick, NJ, USA). The endoscope components were then disconnected and disassembled. Pressure/leak testing was carried out after pre-cleaning in each endoscopy room. Using the containers, we transported the endoscopes and their components to the reprocessing room.
The endoscope and its components were immersed in the detergent solution. The entire endoscope was cleaned with a sponge and brush in detergent solution, including all channels and valves. The detergent was then washed off with tap water and the endoscope and its components were placed randomly into one of the AERs. High-level disinfection was carried out as follows. (1) Control group: 0.55% OPA (Cidex® OPA, Johnson & Johnson), exposure time: 10 minutes at room temperature. (2) Experimental group: 4% TAC (Sencron2®, Bab Gencel Pharma & Chemical Ind. Co., Ankara, Turkey), exposure time: 5 minutes at room temperature.
Following this, the endoscope was rinsed and the channels were flushed with sterile water to remove the disinfectant solution. The endoscope was dried using forced air.
3. Sampling and Culture
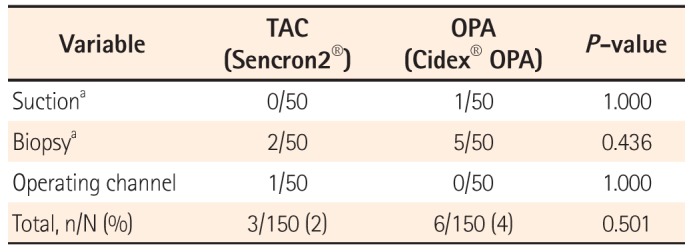
After reprocessing, three culture samples were obtained from each endoscope.
Sample 1:30 mL of sterile saline was flushed through the operating channel and the flow-through was collected into a sterile container (15 mL BD Falcon tube, BD Biosciences, Bedford, MA, USA) at the end of the scope (sample 1). These solutions were filtered through a 0.22 µm cellulose nitrate membrane filter (Falcon Easy Flow 7105, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) under negative pressure, and then, the membrane was immediately spread on a blood agar plate in a sterile manner. Samples 2 and 3: The openings of the suction (sample 2) and biopsy (sample 3) channel were swabbed with sterile saline-soaked cotton swabs (Copan Italia S.p.A., Brescia, Italy), which were then smeared on the surface of blood agar plates. All study samples were tested in the same manner and by the same personnel, who were blinded to the randomization assignment as routine procedure, as was also done with patients' specimens. Blood agar plates were incubated at 35℃ in 5% CO2 for 48 hours. The number of colonies on each plate was counted, and Gram staining for microorganisms was carried out.
4. Statistics
This study was designed to assess the non-inferiority of 4% TAC to 0.55% OPA in terms of successful reprocessing. The sample size for non-inferiority analysis was based on data from the reference "Comparison on the efficacy of disinfectants used in automated endoscope reprocessors: PHMB-DBAC versus Orthophthalaldehyde,"
11 which was calculated with 90% power at a significance level of 5%. And in which 86 endoscope reprocessings (43 endoscopes for each group) were eventually enrolled. Fisher's exact test was used to compare culture rates.
P-values <0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM, Armonk, NY, USA).
Go to :

DISCUSSION
Our results show that the efficacy of TAC was not inferior to that of OPA with regard to high-level disinfection. In addition, there were no complications related to either of these two disinfectants. Based on these results, we conclude that the two disinfectants did not differ in microbiologic or safety aspects. As determined in this study, TAC needs only 5 minutes of contact time at room temperature and is less expensive than OPA (
Table 1). TAC is therefore a good alternative disinfectant to OPA for GI endoscope reprocessing using an AER.
In this study, we used colonoscopes rather than gastroscopes because the former are in greater contact with potential pathogens during the endoscopic examination or procedure. We consistently applied sterile techniques during sample collection, transportation, and drawing in this study. Nine culture-positive results were incidentally found during the study period; these did not occur at the same time and did not represent skin flora. Therefore, we considered all isolated bacteria as having resulted from insufficient disinfection, and not from contamination.
Room temperature varied between 20℃ and 25℃ according to the season and weather. According to the USA Food and Drug Administration (FDA), at least 12 minutes of contact at 20℃ or 5 minutes of contact at 25℃ with 0.55% OPA in an AER is required for high-level disinfection.
13 However, the recommend time required for high-level disinfection with OPA at 20℃ varies worldwide from 5 to 12 minutes.
9 As regular surveillance cultures of endoscopes are performed by the infection control unit at our hospital and the results have been acceptable, we did not regard the fluctuation in room temperature between 20℃ and 25℃ as a big problem.
According to the Spaulding classification system, most flexible GI endoscopes are considered semi-critical devices.
14 The "Multisociety guideline on reprocessing flexible GI endoscopes: 2011" recommends high-level disinfection after use in each patient.
6 The centers for disease control and prevention defines high-level disinfection as the complete elimination of all microorganisms in or on an instrument, except for a small number of bacterial spores.
9
In clinical practice, most pathogen transmissions related to GI endoscopes can be prevented by following established reprocessing guidelines, as we did in this study. Compliance with established reprocessing guidelines is critical, and the ideal endoscopy reprocessing method must be simple, fast, and inexpensive. Similarly, ideal disinfectants, in addition to having established microbiologic efficacy, should also have short contact time requirements and be nonirritating, safe for users, non-corrosive, user-friendly (ready to use and easy to store with stable germicidal activity at room temperature), environmentally friendly, and inexpensive.
Several kinds of disinfectants are available, each with its own advantages and disadvantages. Short contact time is a major consideration in Korea because of the high endoscope turnover. Thus, disinfectants for which shorter contact times are required, such as OPA solution and peracetic acid-plus-hydrogen peroxide formulas, are frequently used in Korea. However, these disinfectants are more expensive than glutaraldehyde solution, which is one of the most commonly used disinfectants in the world.
8 Based on data from a Korean health insurance review and assessment service, the number of colonoscopies plus colonoscopic polypectomies in Korea increased from 1.7 million in 2009 to 2.4 million in 2013.
15 Considering the growing need for GI endoscopy worldwide, the cost of endoscope reprocessing is a major concern. Thus, lowering the medical costs of GI endoscopy is very important. In this respect, use of TAC has several advantages.
This study had several limitations. First, we used a simple flush technique rather than a flush/brush/flush technique to sample the inner surface of the colonoscope channels. This may be a less efficient way to detect microbiota present in channel lumens. However, no method has been established as a standard for assessing the outcome of endoscope reprocessing,
9 and there were no infection-related complications in this study. Second, we used only blood agar plates for bacterial culture and therefore could not directly compare the effects of the two disinfectants with respect to anaerobic bacterial, mycobacterial, viral, or fungal pathogens. However, most viruses have lower innate resistance to disinfectants than bacteria,
9 and both disinfectants have already been approved based on results of official laboratory tests. OPA, approved by the FDA, has been proven to have better microbicidal activity, including mycobactericidal activity, than glutaraldehyde.
9 TAC has been approved in the European Union after passing DGHM (German society for Hygiene and Microbiology) testing. In the test, 4% TAC had a sufficient bacteriostatic and fungicidal effect in 5 minutes against
Staphylococcus aureus,
Enterococcus hirae,
Pseudomonas aeruginosa,
Candida albicans, and
Mycobacterium terrae under conditions similar to those in routine practice (elevated load with 0.3% albumin and 0.3% sheep-erythrocytes) (Supplement 1, Available from:
http://www.irjournal.org/file/Supplement_1.pdf). Third, we did not evaluate the corrosive or caustic effects of the disinfectants on endoscopes and the attached instruments. However, as mentioned earlier, both have already been used in practice for several years after receiving approval. TAC is categorized as a Class IIb (Invasive Medical Device) disinfectant according to "Biocidal Products Directive (BPD) 98/8/EC" that aims to ensure that all biocidal products on sale are safe when used properly, and these products are freely traded within the European Union.
In summary, the efficacy and safety of TAC were not inferior to those of OPA. TAC required a relatively shorter contact time and was less expensive than OPA. Therefore, TAC seems a good alternative disinfectant for GI endoscope reprocessing using an AER.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download