Abstract
Background/Aims
Gastric pathology and Helicobacter pylori (H. pylori) infection among Asian patients with Crohn's disease (CD) are still unclear. We evaluated gastric histologic features and frequency of H. pylori infection in Korean patients with CD.
Methods
Among 492 patients with CD receiving upper gastrointestinal (GI) endoscopic evaluation in 19 Korean hospitals, we evaluated the endoscopic findings and gastric histopathologic features of 47 patients for our study. Histopathologic classification was performed using gastric biopsy tissues, and H. pylori infection was determined using the rapid urease test and histology.
Results
There were 36 men (76.6%), and the median age of patients at the time of upper GI endoscopy was 23.8 years (range, 14.2–60.5). For CD phenotype, ileocolonic disease was observed in 38 patients (80.9%), and non-stricturing, non-penetrating disease in 31 patients (66.0%). Twenty-eight patients (59.6%) complained of upper GI symptoms. Erosive gastritis was the most common gross gastric feature (66.0%). Histopathologically, H. pylori-negative chronic active gastritis (38.3%) was the most frequent finding. H. pylori testing was positive in 11 patients (23.4%), and gastric noncaseating granulomata were detected in 4 patients (8.5%). Gastric noncaseating granuloma showed a statistically significant association with perianal abscess/fistula (P=0.0496).
Conclusions
H. pylori-negative chronic active gastritis appears to be frequent among Korean patients with CD. The frequency of H. pylori infection was comparable with previous studies. An association with perianal complications suggests a prognostic value for gastric noncaseating granuloma in patients with CD.
Crohn's disease (CD) is a chronic IBD of unknown etiology that can involve any site of the gastrointestinal (GI) tract, from the mouth to the anus. Symptomatic gastroduodenal involvement in CD is reported in 1%–5% according to Western literatures.1 However, in contrast to the low frequency of symptomatic gastroduodenal CD, routine upper GI endoscopy identifies microscopic inflammation in up to 70% of Western patients.23
Helicobacter pylori-negative gastritis has been reported in 10%–60% of patients with CD.456 Low infection rates of H. pylori in IBD patients compared with controls have been reported in Western literatures.7891011121314151617
Historically, IBD and CD have been regarded as rare among Asian people. However, in the past few decades, the incidence of IBD, including CD, is steadily increasing in Asia.18192021 The incidence of CD appears to be rising more rapidly than that of UC.19 Therefore, more attention is needed regarding the clinical characteristics of IBD, especially CD in Asian people. There are several differences in the clinical features and prognosis of CD in Asians compared with Western populations, such as male predominance, lower frequencies of isolated colonic disease, and probably a more favorable prognosis.192022 Moreover, genetic backgrounds of Asian IBD patients also were reported to be different from those of Western patients.23 Clinical and genetic differences between Asian and Western patients with CD suggests the possibility of differences in upper GI lesions as well. However, this issue has not been properly investigated in an Asian population. Therefore, in this study, we aimed to characterize gastric lesions in Korean patients with CD in a multicenter study design.
Medical information of 492 patients with CD who underwent upper GI endoscopy in 19 Korean medical institutions was collected for this study. CD was diagnosed based on conventional clinical, radiologic, endoscopic, and histopathologic criteria.1924 Among 492 subjects, 47 patients who met all of the following criteria were finally enrolled: (1) Patients who underwent biopsy for abnormal gastric lesions and/or normal-looking gastric mucosa, and (2) Patients who received rapid urease test (RUT) from both the gastric antrum and corpus. We retrospectively reviewed the patients' medical records, upper GI endoscopic images and histopathology. The following demographic and clinical information was gathered: sex, date of CD diagnosis, date of upper GI endoscopy, presence of upper GI symptoms (epigastric soreness/pain, nausea/vomiting, indigestion, and reflux), perianal abscess/fistula, ileocolonic noncaseating granuloma, medication for CD ever used (5-aminosalicylic acids, thiopurines, and anti-tumor necrosis factor agents), and CRP at the time of upper GI endoscopy. Location and behavior of CD were evaluated using the Montreal classification, excluding upper GI involvement (L4) and perianal disease modifier (p).25 CD activity was evaluated with the CDAI, and was classified as remission (CDAI <150), mild activity (CDAI 150–219), moderate activity (CDAI 220–450), and severe activity (CDAI >450).2627 The study protocol was approved by the Institutional Review Boards of participating institutions.
All upper GI endoscopic evaluations were performed by expert GI endoscopists of the participating institutions. Gross mucosal findings by endoscopy were classified as described previously.28 Biopsies were obtained for pathologic evaluation from abnormal gastric lesions and/or normallooking gastric mucosa at the endoscopists' discretion. Additional biopsies were performed at both the gastric antrum and corpus for RUT. Serologic tests, urea breath test, and culture assay were not used to evaluate H. pylori infection. Histologic features were classified as described previously.5 Briefly, subjects were classified into one of the following categories: (1) H. pylori-negative chronic active gastritis, (2) H. pylori-negative chronic inactive gastritis, (3) H. pylori gastritis, (4) focally enhanced gastritis, and (5) normal.5 Because these histologic diagnoses are not mutually exclusive, the study subjects were classified into category of the most dominant feature. The presence of noncaseating granuloma was also added for groups 1–4 when present. For histopathologic diagnosis of gastric biopsies, electronic files of standard pathologic images for each category were distributed to participating researchers. Standard H&E were performed for biopsy specimens. H. pylori was determined to be positive when RUT and/or histologic evaluation was positive.
Continuous variables were expressed as the median with ranges. Discrete data were expressed as numbers and percentages. Study subjects' demographic and clinical characteristics were compared with regard to H. pylori-positivity and the presence of noncaseating granuloma. For comparative analyses, the Mann-Whitney U test was used for continuous variables, and the chi-squared test or the Fisher's exact test was used for categorical variables, where appropriate. All statistical analysis and calculations were carried out using IBM SPSS Statistics for Windows (version 21.0; IBM Corp., Armonk, USA). A P<0.05 was considered statistically significant.
Among 492 subjects, a total of 47 patients meeting all of the selection criteria were enrolled. These patients had been diagnosed with CD between June 1998 and October 2011. There were 36 men (76.6%), and the median age at diagnosis of CD was 23.1 years (range, 12.0–49.9). Upper GI endoscopies were performed between September 2002 and February 2013. At the time of upper GI endoscopy, the median age of the patients was 23.8 years (range, 14.2–60.5) and the median interval from diagnosis of CD to upper GI endoscopy was 1.6 months (range, 0–168.3).
At the time of upper GI endoscopy, ileocolonic location (80.9%), and non-stricturing, non-penetrating behavior (66.0%) were the dominant phenotypes. Active and/or past perianal abscess/fistula was identified in 23 patients (48.9%). Moderate activity (42.6%) by CDAI was the most common disease activity at upper GI endoscopy. In 22 patients (46.8%), noncaseating granuloma had previously been identified from ileocolonoscopic biopsy specimens. Twenty-eight patients (59.6%) complained of upper GI symptoms. CD medications given in the past to patients included 5-aminosalicylic acids (21 patients, 44.7%), thiopurines (11 patients, 23.4%), and anti-tumor necrosis factor agents (2 patients, 4.3%). In 39 patients (83.0%), CRP was checked at the time of upper GI endoscopy, and the median level was 2.54 mg/dL (range, 0.10–11.18). The characteristics of study subjects are summarized in Table 1.
Erosive gastritis was most common (n=31, 66.0%) feature found on upper endoscopy, followed by normal findings (n=7, 14.9%). H. pylori testing was positive in 11 patients (23.4%). H. pylori-negative chronic active gastritis was most common histopathologic feature (n=18, 38.3%). Noncaseating granuloma was detected in 4 patients (8.5%). The upper GI endoscopic and pathologic findings of study subjects are summarized in Table 2. Representative images of upper GI endoscopy and pathologic features of H. pylori-negative chronic active gastritis with noncaseating granuloma are presented in Fig. 1 and Fig. 2, respectively.
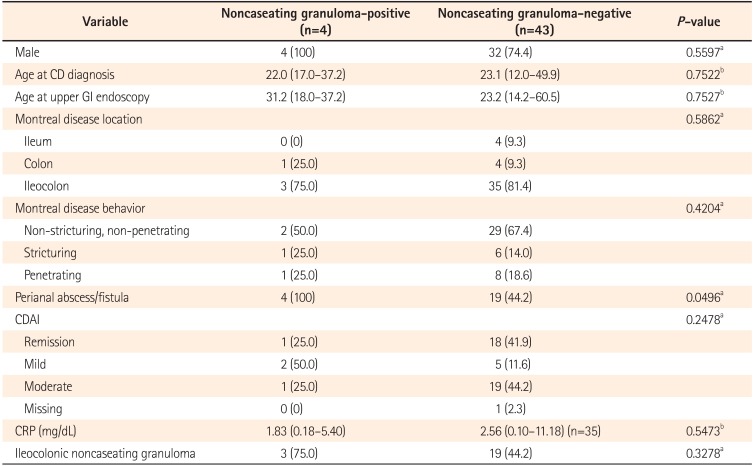
When the association between H. pylori-positivity and various clinical, laboratory, and histologic characteristics was analyzed, there were no variables showing an association with H. pylori-positivity (Table 3). The association between gastric noncaseating granuloma and clinical, laboratory, and histologic characteristics was also analyzed, and gastric noncaseating granuloma was significantly associated with perianal abscess/fistula (P=0.0496, Table 4).
In this pilot study, we identified H. pylori-negative chronic active gastritis in nearly 40%, and H. pylori gastritis in nearly 25%, of Korean patients with CD. Gastric noncaseating granuloma was observed in 8.5% of study subjects and was significantly associated with perianal abscess/fistula.
According to European evidence-based consensus on the diagnosis and management of CD, at the time of CD diagnosis, further investigation to examine the location and extent of disease in the upper GI tract or small bowel is recommended, regardless of the findings at ileocolonoscopy.29 Moreover, in cases of IBD unclassified, upper GI endoscopy could be more useful than imaging studies for establishing the diagnosis of CD, since endoscopic biopsy can document the presence of granulomata or focally-enhanced or focal active gastritis.29
H. pylori-negative chronic active gastritis has been reported to be more common in patients with CD than in the general population. In a recent study with endoscopic biopsy specimens, H. pylori-negative chronic active gastritis was diagnosed in 16.8% of CD patients (35/208), and 1.6% of controls (80/4942), making the diagnosis significantly more frequent in patients with CD (OR, 11.7; 95% CI, 7.5–18.0) than in controls.5 Moreover, among those 21 years of age or younger, H. pylori-negative chronic active gastritis was detected in 26.2% of CD patients (39/149), yielding an OR of 15.1 (95% CI, 9.8–22.9).6 In our study, H. pylori-negative chronic active gastritis was diagnosed in 38.3% of study subjects, which is slightly higher than the frequency reported in recent Western studies.56 This finding needs to be confirmed in a larger-scaled study with controls, evaluated together with the clinical implications of gastric pathologic features, such as an association with prognosis.
Recent laboratory evidence illustrates that H. pylori plays a certain role in regulation of the immune system.30 A possible causative role of H. pylori in the development of autoimmune diseases such as immune thrombocytopenia, and a possible protective role of H. pylori against asthma also suggest interplay between H. pylori and the human immune system.303132 From the epidemiologic point of view, the higher prevalence of IBD in areas with lower rates of H. pylori infection and the rising incidence of IBD together with increased use of eradication therapy in H. pylori endemic areas suggests an association between H. pylori infection and IBD.2033 Several previous Western studies reported low rate of H. pylori infection in patients with IBD as assessed through serologic evaluation,7911131415 or histology and/or RUT.81017 A Japanese study reported 36% H. pylori-positivity by serologic and histologic evaluation among patients with CD (9/25), and a lower H. pylori infection rate among patients with IBD compared with controls (29.4% vs. 52.0%; RR, 0.57; 95% CI, 0.32–1.00).34 Combining multiple studies from Europe, North America, South America, and Japan, a recent meta-analysis reported the lower overall rate of H. pylori infection in patients with IBD compared with controls (27.1% vs. 40.9%; RR, 0.64; 95% CI, 0.54–0.75).33 A previous Korean multicenter study also compared the prevalence of H. pylori infection using urea breath test in 169 patients with UC, 147 with CD, and 316 age- and sex-matched healthy controls.35 They found significantly lower rates of H. pylori infection in both CD (17.7%) and UC patients (32.0%) than in controls (52.5%) (CD vs. controls, P<0.001 and UC vs. controls, P<0.001).35 H. pylori-positivity in our study was 23.4%, which is in between the rates reported in a Korean study35 and a Japanese study.34 However, caution is needed in interpreting and comparing study results, due to differences in the diagnostic methods, study population, and study period.
There have been several studies on the association of noncaseating granuloma with disease severity and clinical outcome of patients with CD. Granulomata observed in the resected intestinal specimens were associated with post-operative endoscopic recurrence and reoperation in patients with CD.363738 In addition, the presence of granulomata within small bowel or colonic surgical specimens of patients with CD was associated with perianal fistula.39 Similarly, epithelioid granulomata noted in endoscopic rectosigmoid biopsies obtained from untreated pediatric patients at initial presentation of CD showed associations with more extensive disease involvement, perianal disease, and increased need for surgery during follow-up, suggesting a worse prognosis.40 In line with those reports, among children, granulomata detected in the GI tract at diagnosis of CD were associated with perianal disease and infliximab treatment during follow-up.41 In our study, noncaseating gastric granulomata were observed in 8.5% of study subjects, which is within the reported frequency of 5%–83% from gastric biopsies in CD.424344 The suggested association between gastric noncaseating granuloma and perianal abscess/fistula from our study needs further confirmation with larger number of cases, including an assessment of the association with other clinical outcomes.
The limitations of our study include its retrospective design, referral center-based study population, and small number of patients. In addition, control subjects without evidence of CD were not investigated for comparison. To evaluate H. pylori , histologic evaluation using special staining such modified Giemsa staining, urea breath test, and serology were not performed. In addition, previous use of antibiotics and proton pump inhibitors at the time of H. pylori evaluation was not properly investigated. However, to avoid possible bias, a standard protocol for evaluating and classifying pathologic features were provided to all participating institutions, and the enrolled cases were selected by a single IBD specialist after thorough evaluation of data. In addition, as far as we are aware, this is the first multicenter study to evaluate both gross and microscopic gastric lesions of Asian patients with CD.
In conclusion, H. pylori-negative chronic active gastritis appears to be common in Korean patients with CD, similar to the frequency seen in Western patients. The H. pylori infection rate was comparable with previous studies. Gastric noncaseating granuloma was associated with perianal abscess/fistula, suggesting an association of granuloma with poor prognosis. A further well-designed prospective study is needed to elucidate the upper GI characteristics of Korean and Asian patients with CD.
Notes
References
1. Turner D, Griffiths AM. Esophageal, gastric, and duodenal manifestations of IBD and the role of upper endoscopy in IBD diagnosis. Curr Gastroenterol Rep. 2009; 11:234–237. PMID: 19463224.

2. Korelitz BI, Waye JD, Kreuning J, et al. Crohn's disease in endoscopic biopsies of the gastric antrum and duodenum. Am J Gastroenterol. 1981; 76:103–109. PMID: 7304545.
3. Cameron DJ. Upper and lower gastrointestinal endoscopy in children and adolescents with Crohn's disease: a prospective study. J Gastroenterol Hepatol. 1991; 6:355–358. PMID: 1912443.

4. Halme L, Kärkkäinen P, Rautelin H, Kosunen TU, Sipponen P. High frequency of helicobacter negative gastritis in patients with Crohn's disease. Gut. 1996; 38:379–383. PMID: 8675090.

5. Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011; 17:39–44. PMID: 20848539.

6. Genta RM, Sonnenberg A. Non-Helicobacter pylori gastritis is common among paediatric patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012; 35:1310–1316.

7. el-Omar E, Penman I, Cruikshank G, et al. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut. 1994; 35:1385–1388. PMID: 7959192.

8. Mantzaris GJ, Archavlis E, Zografos C, Zavos K, Petraki K, Triadaphyllou G. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulfasalazine. Am J Gastroenterol. 1995; 90:1900. PMID: 7572928.
9. Wagtmans MJ, Witte AM, Taylor DR, et al. Low seroprevalence of Helicobacter pylori antibodies in historical sera of patients with Crohn's disease. Scand J Gastroenterol. 1997; 32:712–718. PMID: 9246713.

10. Meining A, Bayerdörffer E, Bastlein E, et al. Focal inflammatory infiltrations in gastric biopsy specimens are suggestive of Crohn's disease. Crohn's Disease Study Group, Germany. Scand J Gastroenterol. 1997; 32:813–818. PMID: 9282974.

11. Parente F, Molteni P, Bollani S, et al. Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel diseases. A cross-sectional study with matching. Scand J Gastroenterol. 1997; 32:1140–1146. PMID: 9399396.

12. Parente F, Cucino C, Bollani S, et al. Focal gastric inflammatory infiltrates in inflammatory bowel diseases: prevalence, immunohistochemical characteristics, and diagnostic role. Am J Gastroenterol. 2000; 95:705–711. PMID: 10710061.

13. Väre PO, Heikius B, Silvennoinen JA, et al. Seroprevalence of Helicobacter pylori infection in inflammatory bowel disease: is Helicobacter pylori infection a protective factor? Scand J Gastroenterol. 2001; 36:1295–1300. PMID: 11761020.
14. Guslandi M, Fanti L, Testoni PA. Helicobacter pylori seroprevalence in Crohn's disease: lack of influence by pharmacological treatment. Hepatogastroenterology. 2002; 49:1296–1297. PMID: 12239929.
15. Triantafillidis JK, Gikas A, Apostolidiss N, Merikas E, Mallass E, Peros G. The low prevalence of helicobacter infection in patients with inflammatory bowel disease could be attributed to previous antibiotic treatment. Am J Gastroenterol. 2003; 98:1213–1214. PMID: 12809861.

16. Prónai L, Schandl L, Orosz Z, Magyar P, Tulassay Z. Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease - antibiotic use in the history does not play a significant role. Helicobacter. 2004; 9:278–283. PMID: 15165265.

17. Sładek M, Jedynak-Wasowicz U, Wedrychowicz A, Kowalska-Duplaga K, Pieczarkowski S, Fyderek K. The low prevalence of Helicobacter pylori gastritis in newly diagnosed inflammatory bowel disease children and adolescent. Przegl Lek. 2007; 64(Suppl 3):65–67. PMID: 18431918.
18. Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum. 2000; 43(Suppl 10):S85–S93. PMID: 11052483.
19. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549. PMID: 17941073.

20. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008; 103:3167–3182. PMID: 19086963.

21. Chuang CH, Lin SH, Chen CY, Sheu BS, Kao AW, Wang JD. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998-2010. Inflamm Bowel Dis. 2013; 19:2815–2819. PMID: 24141711.

22. Park SH, Yang SK, Park SK, et al. Long-term prognosis of Crohn's disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014; 20:488–494. PMID: 24412992.

23. Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:1164–1176. PMID: 21887729.

24. Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998; 114:1161–1168. PMID: 9609752.

25. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753. PMID: 16698746.

26. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976; 70:439–444. PMID: 1248701.
27. Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003; 17(Suppl 2):11–17. PMID: 12786607.
28. Park HK, Kim N, Lee SW, et al. The distribution of endoscopic gastritis in 25,536 heath check-up subjects in Korea. Korean J Helicobacter Up Gastrointest Res. 2012; 12:237–243.
29. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27. PMID: 21122488.

30. Hasni SA. Role of Helicobacter pylori infection in autoimmune diseases. Curr Opin Rheumatol. 2012; 24:429–434. PMID: 22617822.
31. Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008; 198:553–560. PMID: 18598192.

32. Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008; 3:e4060. DOI: 10.1371/journal.pone.0004060. PMID: 19112508.
33. Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010; 16:1077–1084. PMID: 19760778.

34. Furusu H, Murase K, Nishida Y, et al. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn's disease. Hepatogastroenterology. 2002; 49:639–643. PMID: 12063959.
35. Song MJ, Park DI, Hwang SJ, et al. The Prevalence of Helicobacter pylori Infection in Korean Patients with Inflammatory Bowel Disease, a Multicenter Study. Korean J Gastroenterol. 2009; 53:341–347. PMID: 19556840.

36. Yamamoto T. Prevention of recurrence after surgery for Crohn's disease: efficacy of infliximab. World J Gastroenterol. 2010; 16:5405–5410. PMID: 21086556.

37. Simillis C, Jacovides M, Reese GE, Yamamoto T, Tekkis PP. Meta-analysis of the role of granulomas in the recurrence of Crohn disease. Dis Colon Rectum. 2010; 53:177–185. PMID: 20087093.

38. Kotze PG, Saad-Hossne R. Biological therapy for the prevention and treatment of postoperative endoscopic recurrence in Crohn's disease: time for acceptance? Intest Res. 2013; 11:256–260.

39. Denoya P, Canedo J, Berho M, et al. Granulomas in Crohn's disease: does progression through the bowel layers affect presentation or predict recurrence? Colorectal Dis. 2011; 13:1142–1147. PMID: 20860713.

40. Markowitz J, Kahn E, Daum F. Prognostic significance of epithelioid granulomas found in rectosigmoid biopsies at the initial presentation of pediatric Crohn's disease. J Pediatr Gastroenterol Nutr. 1989; 9:182–186. PMID: 2809938.

41. De Matos V, Russo PA, Cohen AB, Mamula P, Baldassano RN, Piccoli DA. Frequency and clinical correlations of granulomas in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2008; 46:392–398. PMID: 18367950.

42. Wagtmans MJ, van Hogezand RA, Griffioen G, Verspaget HW, Lamers CB. Crohn's disease of the upper gastrointestinal tract. Neth J Med. 1997; 50:S2–S7. PMID: 9050325.

43. Loftus EV Jr. Upper gastrointestinal tract Crohn's disease. Clin Perspect Gastroenterol. 2002; 5:188–191.
44. Kefalas CH. Gastroduodenal Crohn's disease. Proc (Bayl Univ Med Cent). 2003; 16:147–151. PMID: 16278730.

Fig. 1
Representative upper gastrointestinal endoscopic images of study subjects (A: patient 1, B-D: patient 2). (A) Multiple aphthous erosions are observed in the anterior wall of the gastric antrum. (B) The opening of a fistula in the anterior wall of the antrum (black arrows) and deformity of the pyloric ring due to active ulcer (arrowheads) are observed. On the posterior wall of the antrum, an ulcer scar is also observed (white arrows). (C) Closer view of ulcer of the pyloric ring (arrows) (D) Closer view of the opening of the fistula (arrows). A fistulous tract between the small bowel and gastric antrum was found at surgery.
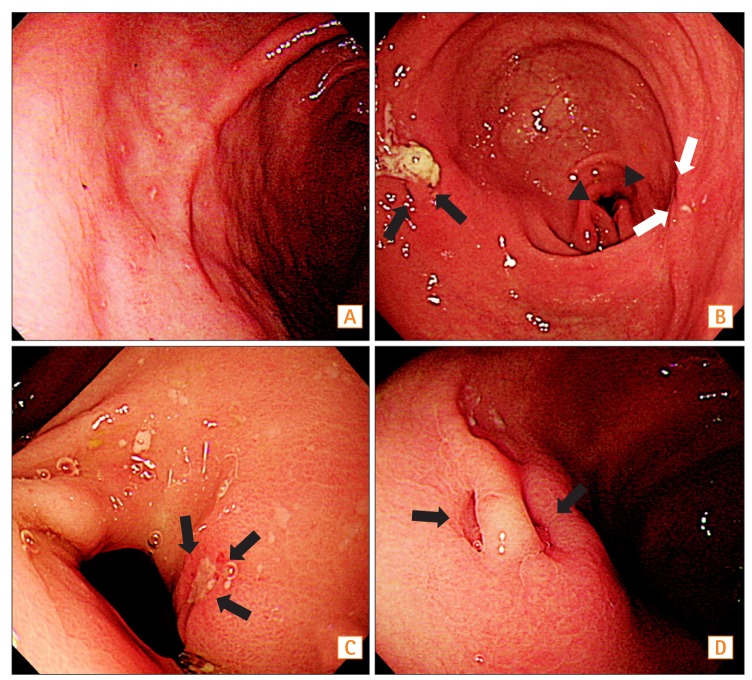
Fig. 2
Histology of biopsy specimens from antral erosions of patient 1. (A) The gastric mucosa showing multifocal aggregates of mixed inflammatory cells in the lamina propria, with multiple crypt abscesses (arrows) and a focal noncaseating granuloma (arrowhead) (H&E, ×100; scale bar=300 µm). (B) The granuloma is of medium size and has no necrosis (arrow) (H&E, ×200; scale bar=120 µm).
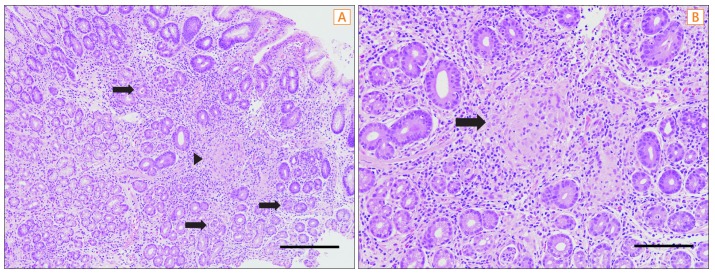
Table 1
Baseline Characteristics of Subjects
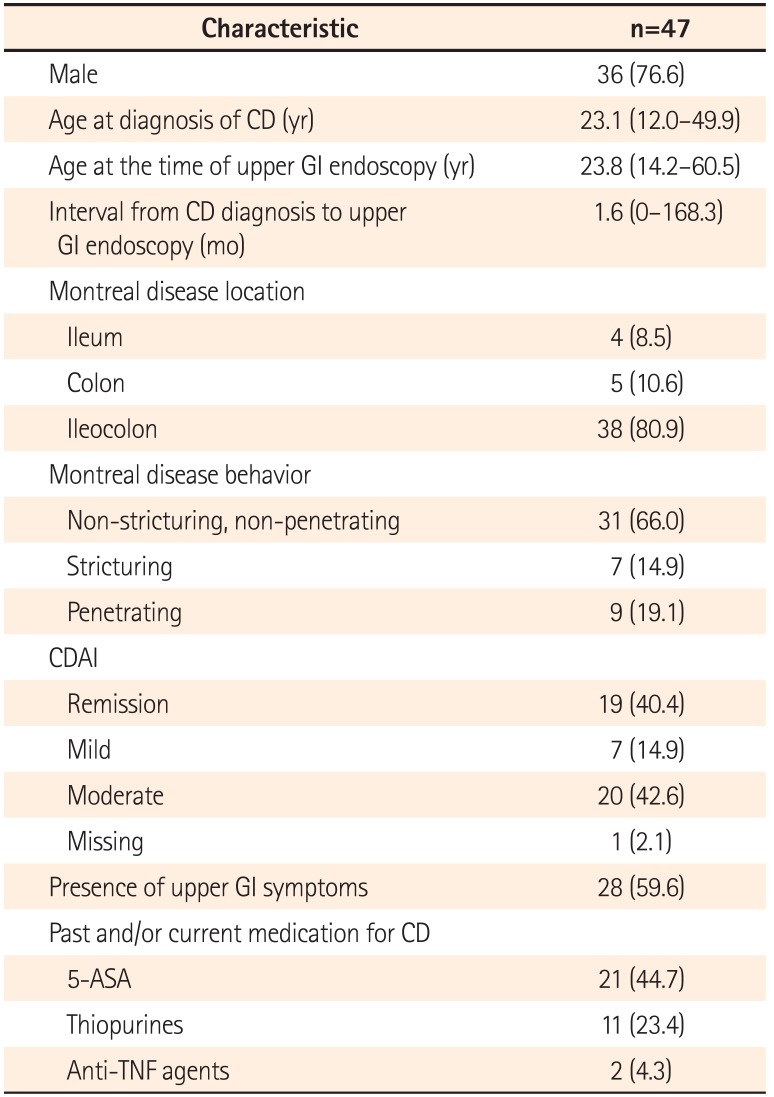
Table 2
Endoscopic and Histologic Findings of Subjects (n=47)
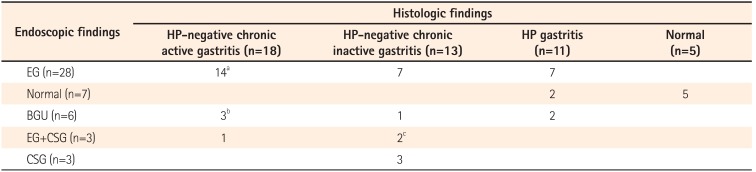
aOne patient was found to have noncaseating granuloma.
bOne patient was found to have noncaseating granuloma, another was found to have combined atrophic change with noncaseating granuloma, and the remaining patient was found to have combined gastric fistula and noncaseating granuloma.
cOne patient was found to have noncaseating granuloma.
HP, Helicobacter pylori; EG, Erosive gastritis; BGU, Benign gastric ulcer; CSG, Chronic superficial gastritis.
Table 3
Association of Helicobacter pylori-positivity With Various Clinical, Laboratory, and Histologic Characteristics
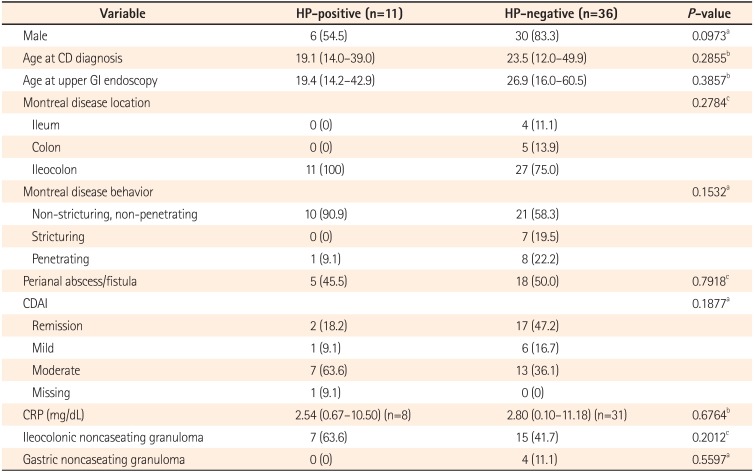
Table 4
Association of Gastric Noncaseating Granuloma With Various Clinical, Laboratory, and Histologic Characteristics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download