INTRODUCTION
Ulcerative colitis (UC) is a chronic relapsing inflammatory disorder characterized by colonic inflammation.
1 5-Aminosalicylate and corticosteroids are conventionally used for treatment of UC.
234 Recently, several papers reported that calcineurin inhibitors and tumor necrosis factor (TNF)-α antagonists are effective treatments for patients with UC refractory to corticosteroids.
56 Particularly, randomized controlled studies demonstrated that, in moderate to severe UC patients, TNF-α antagonists were effective not only for induction and maintenance of remission, but also for achieving endoscopic mucosal healing (MH).
5 Moreover, these trials revealed that achieving endoscopic MH would be a predictive variable of better long-term clinical outcome because of a lower risk of colectomy in UC patients with endoscopic MH.
7 As a result, not only induction of clinical remission, but also achieving endoscopic MH is proposed as the new therapeutic goal for treatment of UC. In clinical practice, however, relapse of UC was found in UC patients despite achieving endoscopic MH. A resent paper also reported that the cumulative relapse rate at 5 years after achieving endoscopic MH was 22% of UC patients.
8 According to those data, it remained controversial whether or not endoscopic MH is the final goal of treatment of UC, because clinical relapse is not infrequent even in patients achieving endoscopic MH. Therefore, identifying the clinical variables predicting relapse in UC patients after achieving endoscopic MH would be important for establishing the optimal therapeutic strategy for maintenance of clinical remission and MH. Hence, the aim of this study was to elucidate the clinical variables predicting relapse in UC patients after achieving endoscopic MH.
DISCUSSION
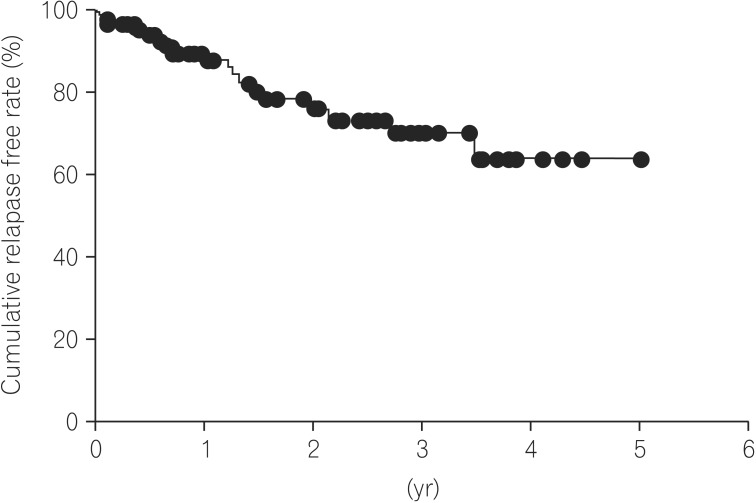
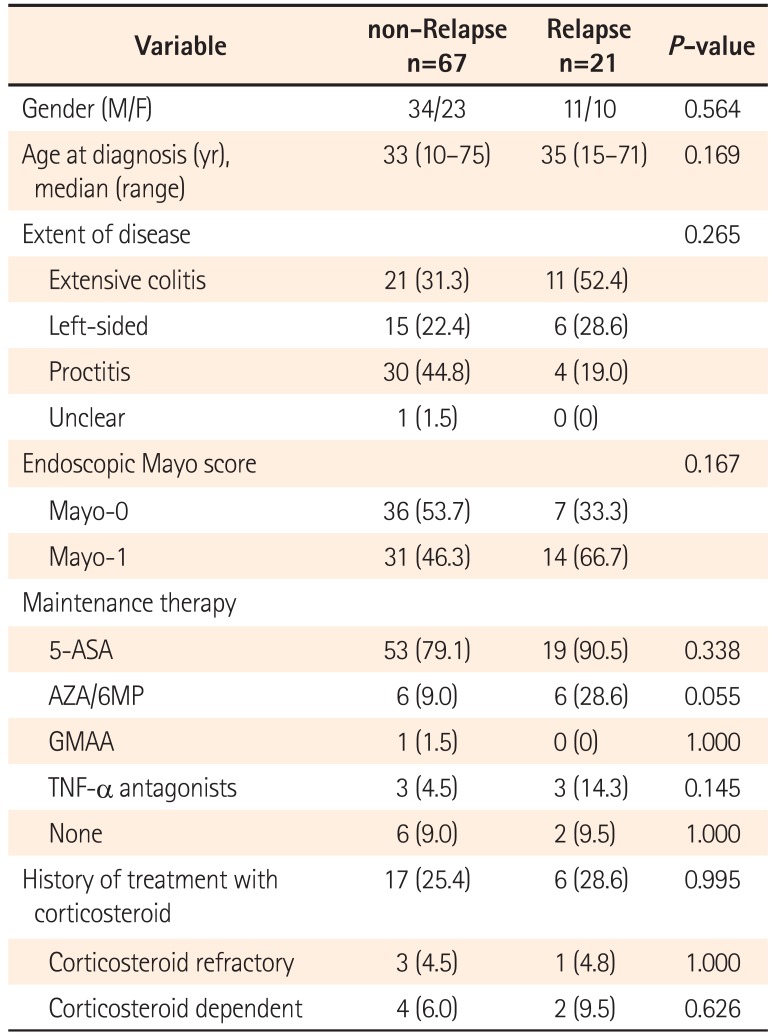
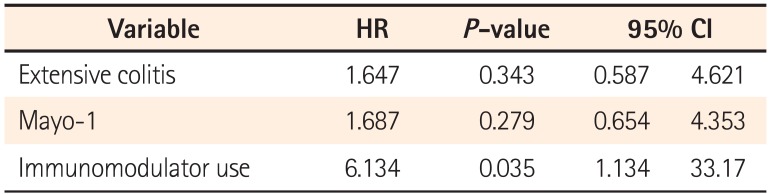
Our current study demonstrated that the cumulative relapse free rate at 5 years of UC patients after achieving endoscopic MH was 63.8%. Moreover, there was no significant difference in the cumulative relapse free rate between the Mayo-0 and Mayo-1 group. Regarding the predictive variable of relapse in UC patients with endoscopic MH, our multivariate analysis revealed that the immunomodulator use was a clinical variable. In UC patients, particularly those requiring immunomodulators, therefore, our data suggest that we should treat toward a higher goal than endoscopic MH, such as histological MH.
It has been well known that endoscopic MH relates to a better long-term clinical-outcome of UC patients.
712 The post hoc analysis of Active Ulcerative Colitis Trials (ACT) 1 and 2 demonstrated that UC patients treated with infliximab who achieved endoscopic MH at week 8 had a lower colectomy rate than those without MH.
7 A recent paper also reported that rapid achievement of endoscopic MH with treatment of tacrolimus was associated with a better remission maintenance time.
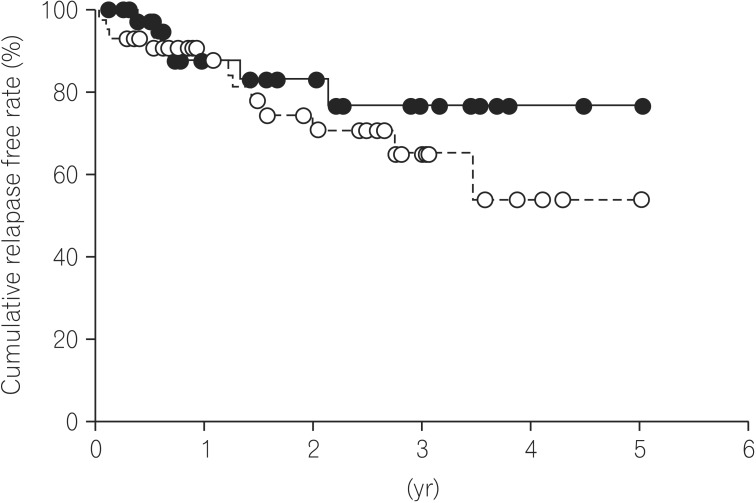
13 Therefore, achieving endoscopic MH has been proposed as the new therapeutic goal for the treatment of UC. However, few papers have reported the long-term outcome of UC patients after achieving endoscopic MH. Previously, Yokoyama et al. reported that cumulative relapse free rates at 5 years of Mayo-0 and Mayo-1 were 78% and 40%, respectively.
8 Moreover, there was a significant difference in the cumulative relapse rate among Mayo-0, -1, -2, and -3 groups although the number of enrolled patients was very small.
8 On the other hand, Ikeya et al. also evaluated the cumulative relapse-free rate between Mayo-0 and Mayo-1 group, and reported that there was no significant difference in prognosis between Mayo-0 and Mayo-1 group.
13 Our data also demonstrated that cumulative relapse free rate at 5 years of Mayo-0 was 76.9% as similar to previous data.
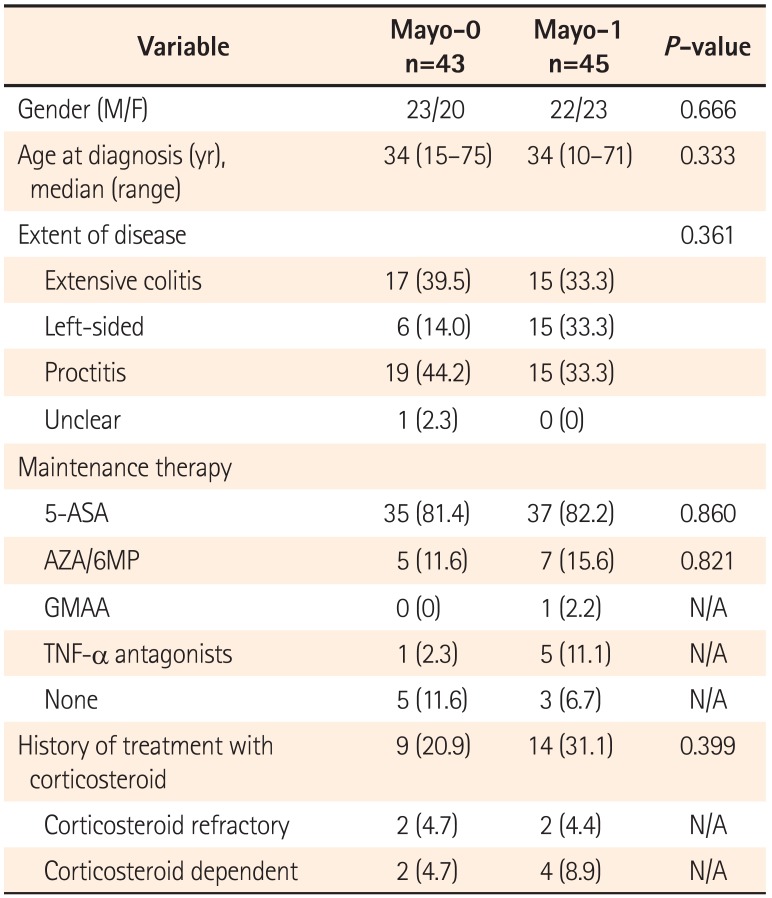
8 In our data, however, there was no significant difference in the cumulative relapse free rate between Mayo-0 and Mayo-1 (
P=0.313). Therefore, it remains controversial whether or not Mayo-1 should be definitely differentiated from Mayo-0 for assessment of endoscopic MH. In either case, endoscopic MH could not be the final goal, but just a milestone in treatment of UC, because the relapse of UC was found not only in the Mayo-1 group, but also in the Mayo-0 group.
Recently, Barreiro-de Acosta et al. reported that a Mayo endoscopic subscore of 1 would be an independent risk factor for relapse during the 6 months of follow-up time after achieving MH.
14 On the other hand, our multivariate analysis revealed that immunomodulator use, but not endoscopic findings, was a predictive variable of relapse for 5 years of follow-up time in UC patient with endoscopic MH. According to those data, endoscopic findings would be associated with clinical relapse in the short-term duration after achieving MH. Regarding relapse over the long-term after achieving MH, however, UC refractoriness requiring immunomodulator for maintenance of remission and MH would contribute to the clinical course of UC patients. Despite achieving endoscopic MH, therefore, we should keep the maintenance treatment deliberately in UC patients who required the treatment with immunomodulator.
It remained unclear whether or not endoscopic MH could be the final goal for treatment of UC. Several papers had also proposed that endoscopic MH is not suitable for the final therapeutic-goal of UC.
151617 Confocal laser endomicroscopy demonstrated that the activity of UC, such as impaired crypt regeneration, persistent inflammation, distinct abnormalities in angioarchitecture, and increased vascular permeability, remained in endoscopically normal colonic-mucosa in patients with UC in remission.
18 According to those data, endoscopic findings alone, even if Mayo-0, are not suitable for assessment of "complete" MH. Recently, therefore, new therapeutic criteria of complete MH defined as histological mucosal healing were proposed.
1819 Particularly, histological evaluation would play an important role in assessment of complete MH, because it is reported that persistent histological inflammation is associated with an increased risk of relapse, hospitalization, and colectomy in UC patients.
17 According to a recent paper by Bryant RV, et al., complete remission, defined as both endoscopic and histological remission, could be associated with long-term better outcome compared with endoscopic remission alone.
19 However, neither endoscopic nor histological remission were perfect for the assessment of complete MH, because 43% of UC patients with both endoscopic and histological remission required corticosteroids, and 12% of those required hospitalization over the 6-year follow-up period.
19 Therefore, establishment of new criteria for assessment of "complete" MH is needed.
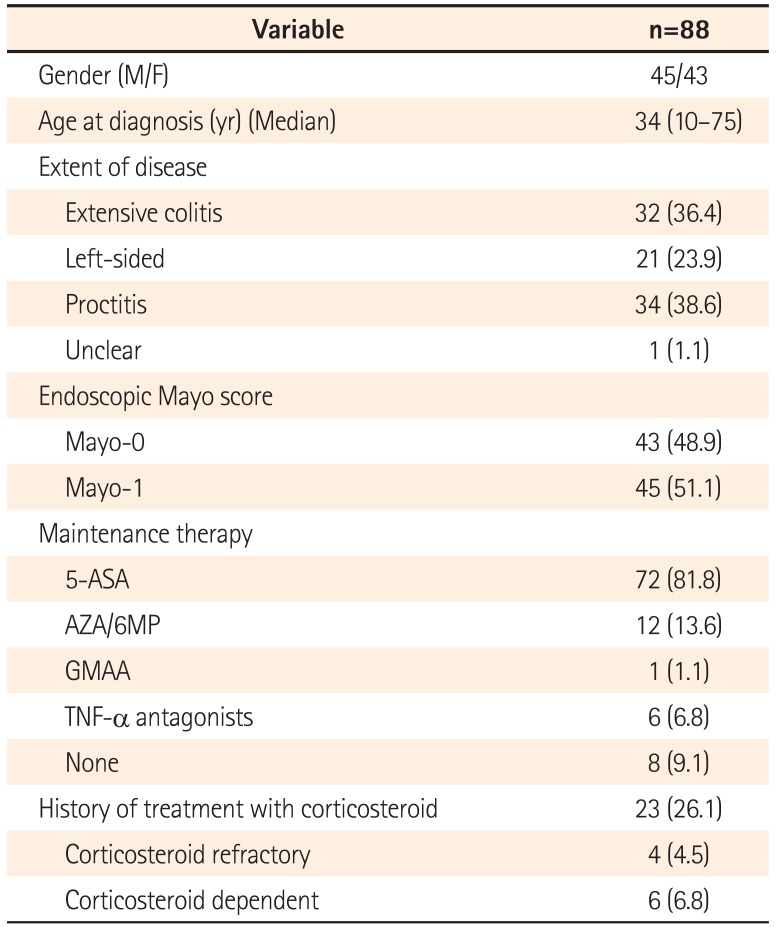
In our study, there were several limitations including small sample size, single center study and retrospective study. Particularly, due to the retrospective study, poor adherence to maintenance therapy or superimposed infection such as Clostridium difficile and cytomegalovirus infections, which would be important variables related to a relapse of UC, could not be analyzed. Moreover, the timing for decision of endoscopic MH in each patient varied. In this regard, our data should be cautiously interpreted and further prospective studies are required with a larger number of enrolled patients.
In conclusion, our data demonstrated that the prognosis of UC patients after achieving endoscopic MH would be based on UC refractoriness requiring immunomodulators, although further studies would be necessary for clarifying the clinical implication of endoscopic- or histologic-MH and evaluating long-term prognostic variables in UC with MH.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download