DISCUSSION
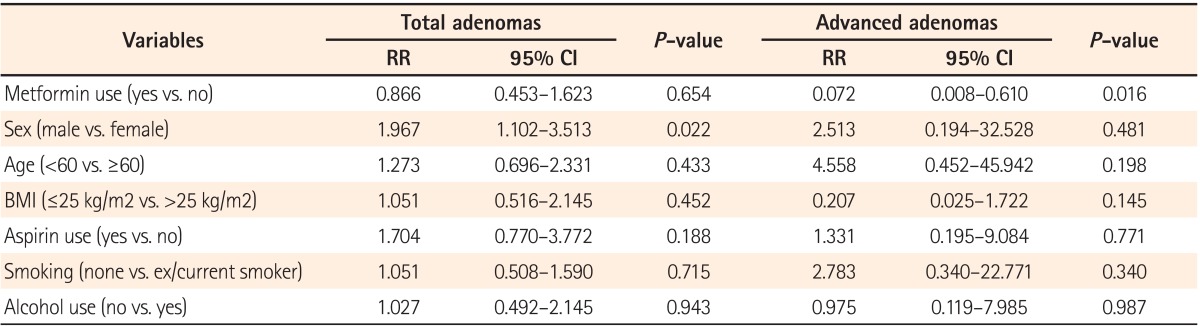
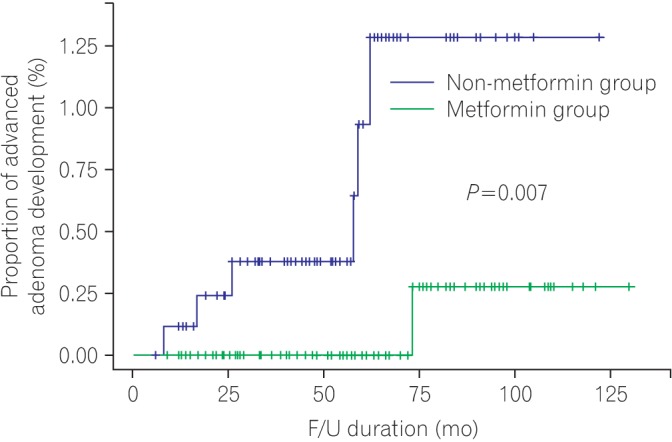
In this study, the use of metformin was found to lower the risk of advanced colorectal adenomas in diabetic patients without previous colorectal cancer. Moreover, metformin use is associated with a decrease in the cumulative development rate of advanced adenomas.
Previous studies have suggested that the use of metformin helps to increase the survival rate in patients with colorectal cancer, as well as lowering the risk of colorectal cancer development.
18,
19,
20 A number of recent studies have also reported that metformin is effective in reducing the risk of colorectal adenomas. In a previous study on patients who underwent screening colonoscopy, 148 out of 405 diabetic patients were receiving metformin, and the incidence of colorectal adenoma was lower in the metformin group (OR, 0.55; 95% CI, 0.34-0.87;
P=0.011).
21 However, the outcome of this study might be limited, because the use of other drugs that might have affected the incidence of adenomas, such as aspirin, among others, was not reported and the groups were not equalized. In a larger-scale study examining 3,105 patients, 912 diabetic patients received metformin, and the risk of adenomas was reduced in the metformin group (OR, 0.74; 95% CI, 0.55-0.98;
P=0.03).
22 However, the metformin group in the study was defined as patients receiving metformin for only a minimum of 1 month before the time of colonoscopy and the medication period was relatively short, an average of 14.7±18.2 months, and unequal. In addition, in a recent study on 240 diabetic patients who underwent surgery due to colorectal cancer, metformin use reduced the incidence of colorectal adenomas (OR, 0.27; 95% CI, 0.100-0.758;
P=0.012).
17 However, the inhibitory effect of metformin required further verification, because the study comprised colorectal cancer patients with a high risk of colorectal adenomas.
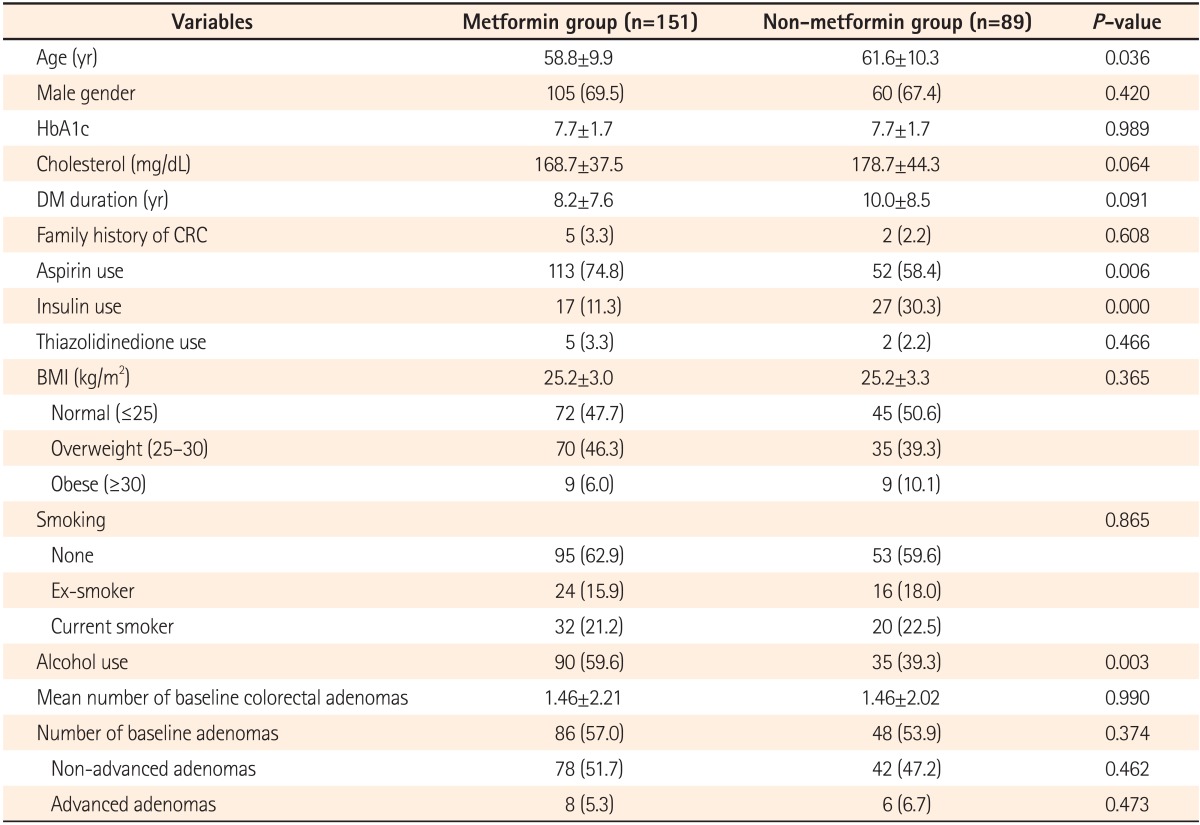
Even though the present study also involved diabetic patients receiving metformin, patient groups were equalized through the removal of their adenomas by means of screening colonoscopy to allow the assessment of the incidence of colonic adenomas according to metformin use, unlike previous studies. Moreover, this study excluded patients diagnosed with colorectal cancer who were at a high risk of developing colorectal adenomas, and examined patients' drug histories including insulin and aspirin use, which could affect adenoma incidence.
Metformin is a widely used anti-diabetic drug and, unlike selective cyclooxygenase-2 inhibitors and other drugs, it has quite a few side effects. For this reason, metformin is an appropriate chemopreventive agent for colorectal cancer. Although the mechanism of metformin that reduces cancer incidence remains unclear, various mechanisms have been proposed. Metformin's mechanisms of action primarily prohibit the cell cycle, cellular growth, and angiogenesis by inhibiting the mTOR pathway.
14 Other metformin-mediated effects include reduction in insulin-like growth factor,
23 hindrance of human epidermal growth factor receptor 2 signaling,
24 and cell cycle arrest,
25 among others. Some studies have suggested that metformin has inhibitory effects on tumor growth through the activation of p53.
26
The exact mechanism responsible for the reduced incidence and cumulative development rates of advanced adenomas in the current study has not yet been clarified. It is anticipated that the results are congruent with the findings of previous studies
27 that metformin reduces the number of aberrant crypt foci (ACF) and dysplastic ACF and suppresses colonic epithelial proliferation. Furthermore, activated p53, which plays an important role in the advanced adenoma stage, seems to be associated. However, metformin had no influence on total adenoma incidence in this study. This outcome contradicts that of a previous study on patients with colorectal cancer.
17 The result is attributed to the fact that it remains unclear at which stage and by what extent metformin influences tumor growth, because metformin may affect the progression of normal tissues to adenoma and cancer throughout all stages, from early to advanced stages. Other possibilities are that the exact dose of metformin actually used might not have been fully reflected in a retrospective study performed based on medical records, and a difference might have been present in the detection rate of adenomas by various endoscopists, although 6 experienced endoscopists performed all procedures. Some studies with relatively small sample sizes have reported that there is no association of metformin use with colorectal adenoma incidence.
28
The exact amount and duration of metformin use for cancer prevention have not yet been clarified. The medication period of metformin varies in previous studies on the effect of metformin on colorectal adenoma and cancer incidence. Lee et al.
19 defined the metformin group as patients with continued use of metformin before and after the diagnosis of colorectal cancer. Garrett et al.
18 compared and analyzed two groups classified according to metformin use at the time of colorectal cancer detection. In both studies, the duration of metformin use varied and was unclear. In a comparative study on colorectal adenoma development and metformin use, there were no standards for the minimum medication period of metformin and the mean duration was 3.91 years.
21 Based on the results of a study
27 that found that the number of ACF was significantly reduced 1 month after metformin use, Cho et al.
22 have recently defined the metformin group as patients who have used metformin for more than a month. The mean duration of metformin was 14.7±18.2 months. No studies have reported that several possible mechanisms for metformin-mediated effects on tumor growth vary in degree according to the duration of metformin use, and the standard dose of metformin sufficient to induce its inhibitory effect on cancer development remains unclear. Moreover, the longer the duration of metformin use, the more likely it is that the medication will suppress the development of colorectal adenomas and cancer. However, no studies have yet investigated the question of a quantitative relationship between the duration of metformin use and tumor suppression. In the present study, the metformin group was defined as patients for whom the medical records showed use of metformin for more than 6 months at a minimum on a regular basis. Although a previous study classified patients with metformin use for a minimum of 1 month as the metformin group, the authors of this study believed that regular use of metformin for 6 months at a minimum is necessary to activate the mechanism of metformin to inhibit colorectal adenomas. In addition, the mean duration of metformin use in the current study was 3.8 years, similar to the longest mean duration in earlier studies (3.9 years).
21
In a previous study on non-diabetic patients, the average number of ACF was considerably reduced (8.78±6.45 vs. 5.11±4.99,
P=0.007) and colonic epithelial proliferation was suppressed in the metformin group at a dose of 250 mg daily for a month compared with the control group. As no changes were observed in the non-metformin group, a low dose of metformin is suggested to be effective in preventing colorectal cancer.
27 Further studies are warranted to clarify the appropriate amount, method, and duration of metformin use to maximize its inhibitory effect on colorectal adenomas or cancer and minimize adverse events. Currently, a double-blind, randomized study on non-diabetic patients with recent polypectomy is in progress in order to compare the effects of metformin on colorectal adenomas between a metformin group at a dose of 250 mg daily and a placebo group for a year.
29
Even though aspirin is known to be effective in reducing colorectal adenoma incidence,
30 it had no influence in the current study. In addition, although insulin and thiazolidinediones have been found to affect adenoma incidence,
31,
32 they showed no effect in this study. However, it is possible that the effects of these anti-diabetic drugs on colorectal adenoma incidence have not been identified, because the dose and duration of these drugs were not accurately examined in this study with a relatively small sample size.
There are several limitations of the present study.
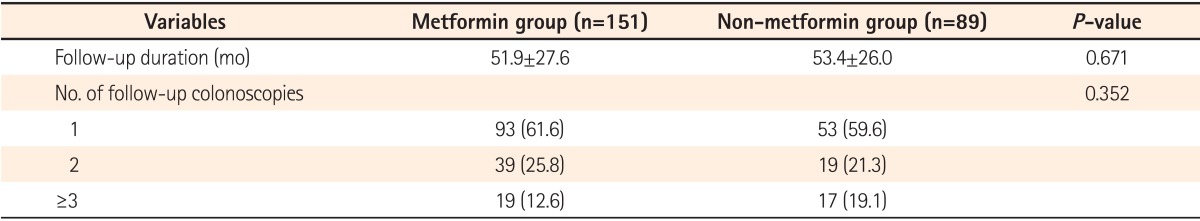
First, randomized allocation was not used to prove the effect of metformin, and the number of surveillance colonoscopies varied from 1 to 3 or more. As there were no differences in the total follow-up duration and the numbers of surveillance colonoscopies between the two groups, those problems could have been partially improved.
Second, errors may be present in data collection in a retrospective study performed based on medical records. Moreover, this study did not examine patients' medical-history in relation to other drugs that could influence the incidence of colonic adenomas, such as aspirin. In particular, we included subjects without knowing their medication period and total dose of metformin before inclusion in this study. Thus, the effect of metformin exposure prior to this research cannot be ignored.
Third, patients with medical records confirming 6 months of regular use of metformin at a minimum were defined as the metformin group. The mean duration was 3.8 years. However, there was an unequal distribution of duration of metformin use, and it is also possible that this period of time was insufficient to verify its inhibitory effect.
Fourth, the quality of colonoscopy was not fully reflected. The degree of quality control including colonoscopy withdrawal time and adenoma detection rate was not sufficiently considered. Although 6 experienced endoscopists performed all procedures throughout the investigation, it is possible that the adenoma detection rate could have been influenced by different endoscopists.
Finally, this research was a single-center study involving a small sample size. The patient group had relatively well-controlled diabetes with a median HbA1c value of 7.7%, and the average periods of DM duration were not long at 8.2 and 10 years for the metformin and non-metformin groups, respectively. For these reasons, the small sample group was insufficient to represent an entire diabetic patient group. Taking into consideration the fact that DM and insulin resistance have significant impacts on adenoma incidence, the incidence rate of adenomas was lower in the current study because a large number of patients with a relatively mild diabetic condition were included. Hence, it is possible that metformin had no inhibitory effect on the total adenoma incidence rate. Therefore, this study was limited in its ability to clearly prove the effect of metformin as a chemopreventive agent for colorectal adenoma incidence in diabetic patients.
To sum up the above study findings, although the authors were unable to clarify the mechanisms of metformin and included diabetic patients only, this study was meaningful in that, unlike other previous studies, it verified the potential of metformin as a preventive agent for patients with an average risk of developing colorectal cancer. We suggest that prospective, randomized, large-scale studies are warranted to further explore the chemopreventive efficacy of metformin on colorectal cancer.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download