Abstract
Background/Aims
Age, sex, gene and life style are modulating risks for colon cancer. Although alcohol intake may impact on colorectal adenoma, clear association has not been established yet. We aimed to investigate effects of alcohol consumption on the characteristics of colorectal adenoma.
Methods
Patients who underwent colonoscopic polypectomy of colorectal adenoma in the department of gastroenterology of Eulji hospital through 2005 to 2012, having both blood tests and ultrasound or abdominal CT examination were enrolled. The alcohol drinking patients were subdivided into normal or abnormal laboratory group, and alcoholic liver diseases group.
Results
212 patients with colorectal adenoma were analyzed; advanced adenoma and multiple adenoma were found in 68 (32.0%) and 79 (37.2%) patients. When compared to the nondrinker group (120/212 patients), the alcohol drinker group (92/212 patients) represented significantly high odds ratios (ORs) for advanced adenoma (OR, 2.697; P=0.002), and multiple adenoma (OR, 1.929; P=0.039). Among alcohol drinker (92 patients), the ORs of advanced adenoma were 6.407 (P=0.003) in alcoholic liver diseases group (17 patients), 3.711 (P=0.002) in the alcohol drinker with abnormal lab (24 patients), and 2.184 (P=0.034), in the alcohol drinker with normal lab (51 patients) compared to nondrinker group.
Although colon cancer has been known to be prevalent in the western countries, the prevalence rate and the mortality rate have been steeply increasing in South Korea in recent days. According to the recent report of Korean National Statistical Office in 2012, the mortality rate of colon cancer is the fourth leading cause of all cancer deaths, after lung cancer, liver cancer, and gastric cancer.1
Foods originated from animals, genetic factors, and IBDs are being known as important risk factors for colon cancer. Life style factors such as alcohol consumption as well as smoking may affect colon cancer, but it is still controversial with a paucity of studies.2,3,4
It has not been clearly established regarding the relationship between alcohol consumption and colorectal adenoma, yet there are several studies describing that alcohol intake impacts on the development of colorectal adenoma.2,5 In order to estimate the amount of alcohol consumption, it was often relying on interviewing but it lacks accuracy and accompanies inter-individual variability considering its nature thereby making it difficult to elucidate exact roles of alcohol intake in reference to colorectal adenoma development. In the current study, we investigated effects of alcohol intake to colorectal adenoma development in patients who underwent colonoscopic polypectomy using not only patients' interview but also results of blood tests and degree of liver diseases by radiologic evaluation.
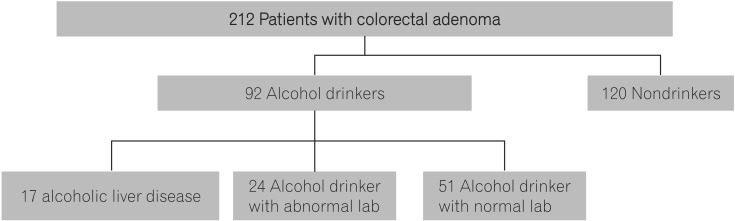
Of patients who underwent colonoscopic polypectomy in the department of gastroenterology of Eulji Hospital through 2005 to 2012, subjects had blood tests and either ultrasound examination or abdominal CT examination within 3 months were enrolled for the study. 328 patients had either abdominal ultrasound examination or abdominal CT. Of them, 212 patients diagnosed with colonic adenoma via histological examinations were enrolled.
Patients who had a history of colonoscopic polypectomy and colon cancer were excluded. Patients with liver cirrhosis due to hepatitis B or hepatitis C, nonalcoholic steatohepatitis, and autoimmune liver diseases were also not included. In addition, patients with liver, gallbladder, biliary tract diseases were excluded since cholecystitis, and cholangitis are able to influence on the level of r-glutamyl transpeptidase (r-GTP) independently from alcohol consumption.
Hyperplastic polyps and cases accompanying colon cancer or IBDs were also excluded.
This study was conducted upon the approval of Clinical Research Ethics Board of the Eulji University College of Medicine and written consent was waived given that this study was performed via retrospective evaluation of medical records (EMCIRB 11-38).
Alcohol consumption per week was evaluated based on the calculation assuming that one bottle of Soju (i.e., Korean vodka; 360 mL per bottle) contains 20% alcohol; this was divided by 7 in order to estimate the average amount of alcohol intake per day.
Alcoholic liver diseases were defined per updated criterion in 2010;6 to be specific, male patients who consumed more than 40 g of alcohol per day more than 5 years and female patients who consumed more than 20 g of alcohol per day more than 5 years were included and then screened based on the results of blood tests results and abdominal ultrasound examination. Alcoholic fatty liver, and alcoholic liver cirrhosis were included for the analysis.6
AST/ALT ratio and levels of r-GTP and mean corpuscular volume (MCV) were evaluated and alcoholic liver diseases were defined in which a patient meets more than two conditions as follow: (1) the ratio of AST/ALT ≥2, (2) increase in r-GTP, and (3) increase in MCV.6 Patients who consumed less than the criteria (i.e., 20 g/day and 40 g/day for female, and male patients, respectively) were just classified as the group with alcohol consumption.
The advanced adenoma was defined as bigger than 10 mm of diameter, villous adenoma, and high grade dysplasia,3 while multiple adenoma was defined in which there are more adenomas than three regardless of location.
IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. All results were expressed mean±SD. Continuous variables were analyzed via the Kruskal-walis test, a non-parametric method whilst categorical variables were examined using the Fisher's exact test. Various variables (e.g., age, sex, smoking, and BMI) were adjusted to find the association between these variables and colorectal adenomas through multivariate logistic regression analysis. A P-value less than 0.05 was considered statistically significant.
Of a total 212 patients, 92 patients were in the group with alcohol consumption while 120 were in the group without alcohol consumption. In the group with alcohol consumption (n=92), 17 patients satisfied the criteria with alcoholic liver diseases but no patient was found for alcoholic liver cirrhosis. In the group with alcohol consumption without alcoholic liver disease (n=75), 24 patients represented abnormal results of the ratio of AST/ALT and levels of r-GTP and MCV whilst 51 patients did not show any noticeable abnormal results. So, the group with alcohol consumption was subdivided into the group with alcoholic liver diseases, the alcohol drinker group with abnormal blood tests results, and the alcohol drinker group with normal blood tests results based on the amount of alcohol intake, and the blood tests results (defined as alcohol drinker group with abnormal lab and the alcohol drinker group with normal lab, respectively; Fig. 1).
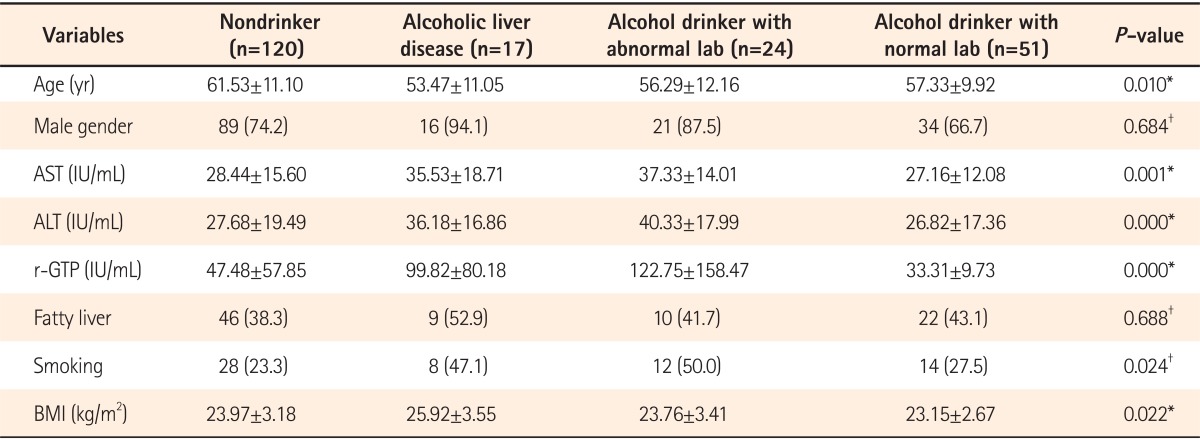
Among the clinical characteristics, the age was relatively higher in the group without alcohol consumption (i.e., the nondrinker group) compared to the other three groups. The proportion of male patients was highest in the group with alcoholic liver diseases (n=16, 94.1%) yet no statistical significance was noted between groups. The fatty liver was shown to be most prevalent in the group with alcoholic liver diseases (n=9, 52.9%) but this was also not statistically different. Smoking was found to be significantly higher in the group with alcoholic liver diseases (n=8, 47.1%) as well as the drinker group with abnormal blood tests results (n=12, 50%; P=0.024). Also BMI was significantly higher in the group with alcoholic liver diseases compared to other groups (P=0.022) (Table 1).
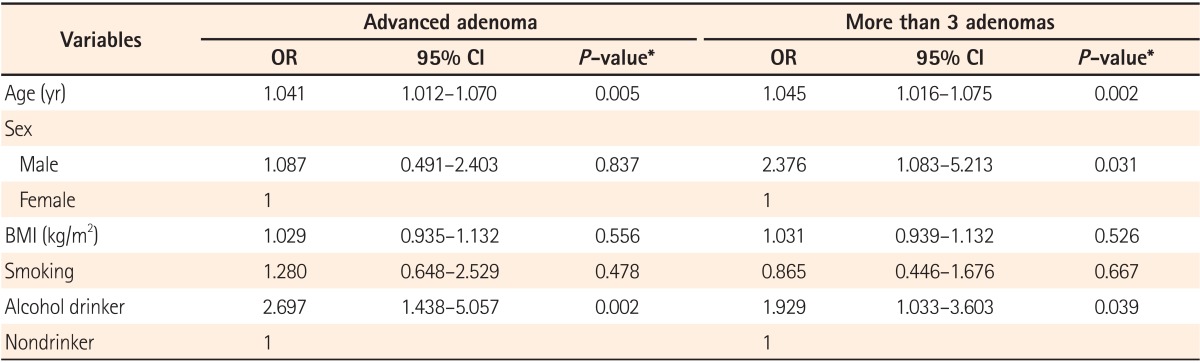
Of a total of 212 patients, advanced adenoma and multiple adenoma were found in 68 patients (32.0%) and 79 patients (37.2%), respectively. Variables affecting on colorectal adenoma including age, sex, BMI, and smoking were adjusted and then direct comparison was made in between the group with/without alcohol consumption. In results, the OR for advanced adenoma was 2.697 (95% CI, 1.438-5.057) in the group with alcohol consumption which was statistically significant compared to that of the group without alcohol consumption (i.e., the nondrinker group; P=0.002). Similarly, the OR for multiple adenoma was also statistically significantly higher in the alcohol consumption group (OR, 1.929; 95% CI, 1.033-3.603; P=0.039) (Table 2).
The group with alcohol consumption was subdivided into the group with alcoholic liver diseases, the alcohol drinker group with abnormal lab, and the alcohol drinker group with normal lab. We analyzed the characteristics of adenoma in the subgroups. The OR for advanced adenoma in these groups was compared to the nondrinker group.
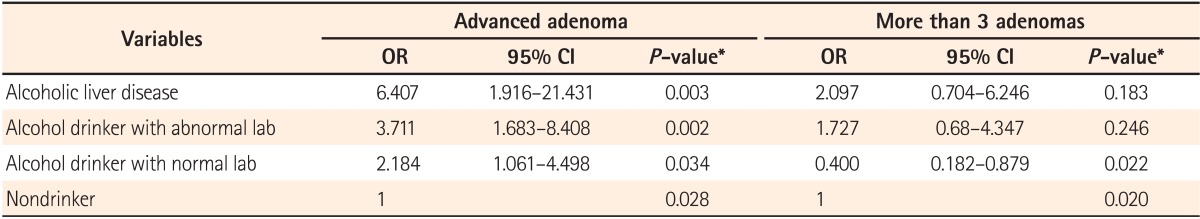
In results, the ORs for advance adenoma in the group with alcoholic liver diseases, the drinker group with abnormal lab, and the drinker group with normal lab were 6.407 (95% CI, 1.916-21.431; P=0.003), 3.711 (95% CI, 1.683-8.408; P=0.002), and 2.184 (95% CI, 1.061-4.498; P=0.034), respectively. These were all significantly higher compared to the nondrinker group. When it comes to multiple adenoma, ORs in the group with alcoholic liver diseases and the alcohol drinker with abnormal lab were higher compared to the nondrinker group, yet no statistical significance was noted (Table 3). Our results indicate that the consumption of alcohol might be one of the important factors influencing the development of advanced adenoma.
Colon cancer is the second leading cause of cancers in the US7 and its incidence rate has been being increased steadily over the last decade in South Korea as well. Fortunately, by virtue of advances in medical technologies as well as establishment of national cancer control program systems, the survival rate of colon cancer is improving, but the mortality rate of this cancer is still the fourth leading cause of all cancer deaths (16.3 deaths per 100,000) in South Korea, after lung cancer, liver cancer, and gastric cancer based on the recent report of Korean National Statistical Office in 2012.
Colorectal adenoma is a prodromal change of colon cancer and detection and elimination of this precancerous lesion has been considered as the most effective preventive method to minimize development, morbidity, and mortality of colon cancer on the adenoma-carcinoma sequence theory.8,9
Risk factors of colon cancer include alcohol intake, smoking, high calorie food intake, and obesity and a number of cohort studies have been done in order to elucidate their association with colon cancer. Smoking and high intake of meats have been known to increase risks of colon cancer. Yang et al.10 demonstrated that the group who consumes high calorie diets and dense animal proteins represents higher risk of colon cancer.
With increase in alcohol consumption, multiple studies have been done regarding the association between alcohol and colorectal adenoma. But the results of studies demonstrated inconsistent outcomes.
Shin et al.3 showed chronic intake of alcohol, more than 28 years, provides higher risk for advanced adenoma and multiple adenomas, indicating that alcohol intake plays a role in colorectal carcinogenesis.
Lieberman et al.11 suggested that excessive alcohol consumption might be a risk factor for colon cancer and alcoholic beverages such as wine and beers may induce rectal adenomas.12 In addition, Morimoto et al.13 reported that alcohol intake is related with increase in risk of adenomas in the patients with polyps and heavy whisky drinking resulted increase in risk of adenoma in male subjects.14 Also there are some reports about colon cancer and alcohol consumption. Shimizu et al.15 addressed the positive correlation between alcohol intake and colon cancer in both male and female. The other study, analyzed 8 different cohort studies regarding the association between alcohol intake and colorectal cancer, suggested that excessive alcohol intake may increase the proportion of colon cancer.16
In contrast, Shrubsole et al.4 reported regular alcohol intake is weakly associated with hyperplastic polyps, whilst Pedersen et al.17 showed wine consumption is not related with colon cancer incident. In other study, alcohol intake was not associated with colon cancer risk.18 These reports have not been inconclusive in the association between development of adenoma and alcohol intake until now.
Our study is significant because results herein indicate the positive correlation between alcohol consumption and occurrence of advanced adenoma in the alcohol drinking groups. The exact mechanisms regarding alcohol intake and their impacts on colorectal adenoma and possibly colon cancer are not completely elucidated yet, but it is possible that alcohol hinders folic acid absorption or inhibits enzymes responsible for folic acid synthesis thereby causing folic acid deficiency in colon and rectum and eventually impacting colon carcinogenesis.19
Methylenetetrahydrofolate reductase (MTHFR), a key enzyme for folic acid synthesis, affects on DNA methylation as well as synthesis hence play a role in folic acid synthesis. Of MTHFR polymorphisms, MTHFR C677TT has been known to interact with folic acid and elevated colon cancer risk in which folic acid was deficient.
In addition, intestinal microflora possesses high alcohol dehydragenase activity hence alcohol can be oxidized in the colon and eventually produces high concenturation of acetaldehydes. Further, alcohol may inhibit tumor immune surveillance, impact on DNA repair, change the composition of biliary fluid, and induce inhibition of hepatic cytochrome P450 enzyme which is able to activate other carcinogens.20
In this study, we investigated the association between alcohol intake and colorectal adenoma incidence; age, sex, BMI, and smoking were adjusted to make a direct comparison for colorectal adenoma properties with alcohol consumption. In the group with alcohol drinking, the numbers of advanced adenoma as well as multiple adenoma were significantly higher compared to that of the nondrinker group.
In the previous study, Shin et al.3 compared the occurrence rate of adenoma depending upon their location in reference to alcohol intake and demonstrated similar results.
In our study, the group with alcohol drinking were subdivided into more specified groups based on lab and radiological evaluation of liver in order to find individual effects of alcohol intake on colorectal adenoma formation depending on liver function.
We demonstrated that the proportion of advanced adenoma was significantly higher both in the group with alcoholic liver diseases and with abnormal lab group. The result suggest that magnitude of alcohol effects on individual liver might be more important than the absolute amount of alcohol intake in adenoma development, meaning the more damage by alcohol, the higher rate of adenoma incidence and progression.
This study has limitation as it was performed via retrospective evaluation for patients only with colorectal adenoma, and was not included early colon cancer and colon cancer patients. In future, large cohort studies are warranted to strengthen the hypothesis of alcohol as risk factor of colon cancer, and clarify the genetic factors making individuals more susceptible for advanced adenoma in response to alcohol consumption.
Notes
References
1. Statistics Korea. Annual report on the cause of death statistics in 2011: nationwide. Daejeon: Statistics Korea;2012.
2. Bardou M, Montembault S, Giraud V, et al. Excessive alcohol consumption favours high risk polyp or colorectal cancer occurrence among patients with adenomas: a case control study. Gut. 2002; 50:38–42. PMID: 11772965.

3. Shin A, Hong CW, Sohn DK, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am J Epidemiol. 2011; 174:552–562. PMID: 21791710.

4. Shrubsole MJ, Wu H, Ness RM, Shyr Y, Smalley WE, Zheng W. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am J Epidemiol. 2008; 167:1050–1058. PMID: 18304959.

5. Rueda M, Robertson Y, Acott A, et al. Association of tobacco and alcohol use with earlier development of colorectal pathology: should screening guidelines be modified to include these risk factors? Am J Surg. 2012; 204:963–967. PMID: 23040696.

6. Li YM, Fan JG, Wang BY, et al. Guidelines for the diagnosis and management of alcoholic liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010;18:167-170). J Dig Dis. 2011; 12:45–50. PMID: 21276208.

7. Mayer RJ. Gastrointestinal tract cancer. In : Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. Volume 1. 18th ed. New York: McGraw-Hill Professional;2011. p. 764–776.
8. Rider JA, Kirsner JB, Moeller HC, Palmer WL. Polyps of the colon and rectum; their incidence and relationship to carcinoma. Am J Med. 1954; 16:555–564. PMID: 13148199.
9. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993; 329:1977–1981. PMID: 8247072.

10. Yang MH, Rampal S, Sung J, et al. The association of serum lipids with colorectal adenomas. Am J Gastroenterol. 2013; 108:833–841. PMID: 23545715.

11. Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003; 290:2959–2967. PMID: 14665657.

12. Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps--a case control study. Eur J Nutr. 2002; 41:35–43. PMID: 11990006.

13. Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002; 11:1012–1018. PMID: 12376501.
14. Honjo S, Kono S, Shinchi K, Imanishi K, Hirohata T. Cigarette smoking, alcohol use and adenomatous polyps of the sigmoid colon. Jpn J Cancer Res. 1992; 83:806–811. PMID: 1399817.

15. Shimizu N, Nagata C, Shimizu H, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. Br J Cancer. 2003; 88:1038–1043. PMID: 12671701.

16. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004; 140:603–613. PMID: 15096331.

17. Pedersen A, Johansen C, Gronbaek M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut. 2003; 52:861–867. PMID: 12740343.

18. Ye W, Romelsjo A, Augustsson K, Adami HO, Nyren O. No excess risk of colorectal cancer among alcoholics followed for up to 25 years. Br J Cancer. 2003; 88:1044–1046. PMID: 12671702.

19. Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci. 2005; 96:535–542. PMID: 16128738.

20. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010; 138:2029–2043. PMID: 20420944.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download