Abstract
Background/Aims
Given the characteristic procedures involved in the endoscopy unit, the spread of pathogens is much more frequent in this unit than in other environments. However, there is a lack of data elucidating the existence of pathogens in the endoscopy unit. The aim of this study was to detect the presence of possible pathogens in the endoscopy unit.
Methods
We performed environmental culture using samples from the endoscopy rooms of 2 tertiary hospitals. We used sterile cotton-tipped swabs moistened with sterile saline to swab the surfaces of 197 samples. Then, we cultured the swab in blood agar plate. Samples from the colonoscopy room were placed in thioglycollate broth to detect the presence of anaerobes. After 2 weeks of culture period, we counted the colony numbers.
Results
The most commonly contaminated spots were the doctor's keyboard, nurse's cart, and nurse's mouse. The common organisms found were non-pathogenic bacterial microorganisms Staphylococcus, Micrococcus, and Streptococcus spp.. No definite anaerobe organism was detected in the colonoscopy room.
Conclusions
Although the organisms detected in the endoscopy unit were mainly non-pathogenic organisms, they might cause opportunistic infections in immunocompromised patients. Therefore, the environment of the endoscopy room should be managed appropriately; moreover, individual hand hygiene is important for preventing possible hospital-acquired infections.
A hospital-acquired infection can prolong the hospitalization period of inpatients and increase medical costs. In addition, a severe infection can endanger the life of a patient.1 Therefore, it is imperative to prevent such infections. The most common route of hospital-acquired infection was found to be the hands of the healthcare staff.2 It has recently been revealed that pathogens are often transmitted through computer keyboards and mouses, which are collectively used by healthcare workers because of the computerization of hospital records.3 Given the characteristics of a gastrointestinal endoscopy room, pathogens can spread more often through the hands of healthcare staff who are easily exposed to body fluids of patients than through any other infection routes. Hence, it is thought that a variety of pathogens are likely to exist on the surface of the equipment inside the endoscopy room.4 However, to the best of our knowledge, no study has been conducted on the presence of pathogens in the endoscopy room so far.
Therefore, this study aimed to confirm the presence of pathogens in an endoscopy unit by performing environmental culture on the surfaces of equipment, which are very often touched by doctors and nurses.
Environmental samples were collected from endoscopy rooms of Keimyung University Dongsan Medical Center and Kyungpook National University Hospital in April and August 2013. In April 2013, environmental samples were collected from 3 endoscopy rooms of Keimyung University Dongsan Medical Center, one time each in the morning and afternoon. In August 2013, samples were collected one time each in the morning and afternoon from 3 endoscopy rooms of Keimyung University Dongsan Medical Center and another 2 endoscopy rooms of Kyungpook National University Hospital. Among the 3 endoscopy rooms of Keimyung University Dongsan Medical Center, one is mainly used for therapeutic endoscopy procedures, while the other 2 are used for diagnostic endoscopy procedures, where upper gastrointestinal endoscopy is usually performed in the morning and colonoscopy, in the afternoon. In April 2013, environmental samples were collected from a total of 17 surfaces including a doctor's computer keyboard, doctor's computer mouse, upper side of the backrest of the doctor's chair, nurse's cart, upper side of the backrest of the nurse's chair, nurse's computer mouse, lighting switches, telephones, and a storage drawer of endoscope accessories. In August 2013, another set of samples was collected from a total of 8 surfaces where bacterial isolates were identified: a doctor's computer keyboard, doctor's computer mouse, upper side of the backrest of a doctor's chair, endoscope handle, endoscopic keyboard, oxygen cannula connector, nurse's cart, and nurse's computer mouse. Each endoscopy room was staffed with doctors and nurses that worked 2 shifts: the morning shift and afternoon shift. All environmental samples including those from the surface of the endoscope handle were collected after endoscopy was performed. The study protocol was approved by the respective Institutional Review Board of the Keimyung University Dongsan Medical Center and Kyungpook National University Hospital involved in this study.
To collect the samples, we used sterilized cotton swabs moistened with sterile saline and rolled the swabs on the surfaces of the environments (Fig. 1A). The collected samples were cultured on blood agar plates (Fig. 1B). Those samples collected from the colonoscopy room were once again put in thioglycollate broth (Fig. 1C). The blood agar plate is often used to check for a hospital-acquired infection, while the thioglycollate broth is used to check for the presence of anaerobic organisms in the samples. The samples from the colonoscopy room were placed in the thioglycollate broth because of the possibility of transmission of anaerobes during colonoscopy.5 After 2 weeks of culturing, we identified and counted the colony-forming units (CFUs) in the samples (Fig. 1D).6
After we collected and analyzed a total of 197 environmental samples, we found that the doctor's computer keyboard had the highest colony count, 974 CFUs, in the endoscopy room, followed by the nurse's cart (918 CFUs) and nurse's computer mouse (764 CFUs). On further breaking down the bacterial isolates according to species, Staphylococcus spp. was the species with the highest number of CFUs, followed by Micrococcus spp. and Streptococcus spp. However, no anaerobic organism was detected in the samples. The culture of the sample from the doctor's computer keyboard had 679 CFUs of Staphylococcus spp., 224 CFUs of Micrococcus spp., 37 CFUs of Bacillus spp., and 8 CFUs of Streptococcus spp.; the nurse's cart had 376 CFUs of Streptococcus spp., 291 CFUs of Staphylococcus spp., 122 CFUs of Micrococcus spp., and 89 CFUs of Bacillus spp.; the nurse's computer mouse had 487 CFUs of Streptococcus spp., 204 CFUs of Staphylococcus spp., 37 CFUs of Bacillus spp., and 35 CFUs of Micrococcus spp.; and the endoscopy keyboard had 305 CFUs of Staphylococcus spp., 146 CFUs of Micrococcus spp., 44 CFUs of Streptococcus spp., and 40 CFUs of Bacillus spp. A total of 502 CFUs of bacterial isolates were observed on the culture of the sample from the backrest of the doctors' chair: 275 CFUs of Staphylococcus spp., 146 CFUs of Micrococcus spp., 40 CFUs of Bacillus spp., and 22 CFUs of Streptococcus spp. A total of 357 CFUs of bacterial isolates were observed on the culture of the sample from the surface of the oxygen cannula connector: 148 CFUs of Streptococcus spp., 122 CFUs of Staphylococcus spp., 40 CFUs of Micrococcus spp., and 12 CFUs of Bacillus spp. A total of 298 CFUs were found on the culture of the sample from the endoscopic handle: 137 CFUs of Micrococcus spp., 115 CFUs of Staphylococcus spp., 41 CFUs of Bacillus spp., and 1 CFU Streptococcus spp. Further, a total of 180 CFUs were found on the culture of the sample from the doctor's computer mouse: 112 CFUs of Staphylococcus spp., 37 CFUs of Micrococcus spp., 10 CFUs of Bacillus spp., and 9 CFUs of Streptococcus spp. (Fig. 2). These results indicate that more colonies were formed on cultures from samples taken from surfaces most often touched by doctors and nurses. Although the species of bacterial isolates may differ depending on each sampling surface, the study failed to detect any definite pathogenic bacterial microorganism including Clostridium difficile,7
Helicobacter pylori, Staphylococcus aureus, and Enterococcus, whose existence was suspected before the test.
Based on the results of the analysis of bacterial isolates from the cultures of samples from each endoscopy room, in the therapeutic endoscopy rooms where environmental culture was done twice, the surface of a nurse's cart had the highest colony count (204 CFUs), followed by the oxygen cannula connector (134 CFUs), and doctor's computer mouse (120 CFUs). On categorizing the findings on the basis of the species found on each sampling surface, the nurse's cart was found to have 169 CFUs of Streptococcus spp., 31 CFUs of Bacillus spp. and 3 CFUs of Micrococcus spp.; the surface of an oxygen cannula connector had 125 CFUs of Streptococcus spp., 5 CFUs of Staphylococcus spp., and 4 CFUs of Micrococcus spp.; and the doctor's computer keyboard had 68 CFUs of Staphylococcus spp., 35 CFUs of Micrococcus spp., 4 CFUs of Bacillus spp., and 2 CFUs of Streptococcus spp. (Fig. 3A). A total of 6 environmental cultures were prepared form samples collected from the diagnostic endoscopic rooms where upper gastrointestinal endoscopy was conducted. The culture of the sample from the nurse's computer mouse had the highest colony counts (563 CFUs), followed by the surface of a nurse's cart (390 CFUs), and endoscopic handle (183 CFUs). With regard to the species found on each sampling surface, the nurse's computer mouse had the highest CFUs of Streptococcus spp. (143 CFUs), followed by Micrococcus spp. (9 CFUs), and Bacillus spp. (7 CFUs). The surface of the nurse's cart had the highest CFUs of Streptococcus spp. (201 CFUs), followed by 113 CFUs of Staphylococcus spp., 29 CFUs of Micrococcus spp., and 16 CFUs of Bacillus spp. The endoscopic handle had the highest CFUs of Micrococcus spp. (93 CFUs), followed by 59 CFUs of Staphylococcus spp., 21 CFUs of Bacillus spp., and 10 CFUs of Streptococcus spp. (Fig. 3B). The environmental culture was performed 6 times using the samples from the diagnostic endoscopic rooms where colonoscopy was usually conducted. The doctor's computer keyboard had the highest CFUs (571 CFUs), followed by the surface of the nurse's cart (324 CFUs), and the backrest of the doctor's chair (279 CFUs). If we categorize the findings according to the species found for each sampling surface, the doctor's computer keyboard had the highest CFUs of Staphylococcus spp. (429 CFUs), followed by Micrococcus spp. (126 CFUs), Bacillus spp. (12 CFUs), and Streptococcus spp. (1 CFU). The surface of the nurse's cart had 178 CFUs of Staphylococcus spp., 90 CFUs of Micrococcus spp., 42 CFUs of Bacillus spp., and 6 CFUs of Streptococcus spp. (Fig. 3C).
As hospital-acquired infections can critically endanger the health of inpatients, many studies have been conducted on finding ways to reduce the prevalence of hospital-acquired infections and the subsequent death rate.8 Recently, it was reported that the most common infection route was the hands of healthcare staff, so the hand hygiene of doctors and nurses is now an important issue.4 However, a variety of electronic devices are installed in almost every corner of a hospital, such as the outpatient ward and examination rooms, owing to the computerization of a hospital system and the development of test equipment, e.g., artificial respirators in the intensive care units, computer-aided prescription systems for doctors and nurses, endoscopes, and ultrasonography systems. Because this equipment can become a potential reservoir of bacteria, they can easily become a transmission route of hospital-acquired infections, which often occur when immunocompromised patients are undergoing a test or a procedure in a hospital. Given that the awareness of such potential risks still remains low, more studies need to be conducted in this regard.
Many people visit a gastrointestinal endoscopy room where many endoscopic surgeries are conducted daily, so these areas can become transmission routes of pathogens because of the high risk of exposure to body fluids of patients.9 Therefore, this study aimed to assess the environment of an endoscopy room as a potential source of hospital-acquired infections and to determine countermeasures to prevent them.
The doctor's keyboard was found to have the highest CFUs, and this could be ascribed to poor hand hygiene, indicating that doctors did not thoroughly wash their hands after coming into contact with a patient or that they did not wear gloves when they were seeing a patient and tended to touch a keyboard or a mouse immediately after coming into contact with the patient. In addition, the nurse's computer mouse and the endoscopic keyboard were other areas where a multitude of bacteria isolates were detected and possibly for the same reasons. Therefore, doctors and nurses need to sterilize or cleanse their hands before touching a computer right after an endoscopic surgery or before contacting a patient, change their gloves when dealing with each patient, and sterilize the surrounding environment with a disinfectant. According to the study by Appelgate et al. on sterilization of equipment and environmental hygiene in an operating room, regular disinfection of the main lighting system, Bovie, hemostats, pulse oximeters, tables, anesthesia machine, carts, telephones, keyboards, and other areas that healthcare providers often touch can improve the level of environmental hygiene and the overall disinfection rate inside the operating room.10
With regard to the species of bacterial isolates, the cultures of the samples from doctor's computer keyboard and mouse, backrest of the doctor's chair, and endoscopic handles were found to have the highest CFUs of Staphylococcus spp. followed by Micrococcus spp. In contrast, the nurse's cart, computer mouse, and oxygen cannula connectors had the highest CFUs of Staphylococcus spp. followed by Streptococcus spp. This indicates that the species in bacterial isolates in the samples from various surfaces may vary depending on occupational groups most frequently in contact with those surfaces. However, as each species of bacterial isolates is a normal flora, which is commonly found on the hands, it is thought that the identified species of bacteria did not show any clinical significance. The most commonly isolated species, Staphylococcus spp., is methicillin-resistant and methicillin-susceptible, while Streptococcus spp., an unspecified alpha hemolytic, was classified as an environmental contaminant as Micrococcus spp., which can be isolated from the surrounding environment and the mouth, hands, and skin of humans. In addition, the rarely isolated Enterococcus was also confirmed to be vancomycin-susceptible, and the gram-negative bacillus was classified not as Pseudomonas or Acinetobacter spp. but as a non-fermenter that can be isolated from the environments such as water systems and hospital instruments.11
In the meantime, the study failed to observe any significant differences in the counts and species of bacterial isolates in the endoscopy rooms, and no definite species of anaerobic bacteria was identified in the endoscopy rooms where diagnostic endoscopy procedures were performed in the afternoon. Given the results that almost no colony of Escherichia coli, the most dominant strain among enteric bacteria, was found in the samples, it was assumed that the species of bacteria contaminating the environment in the endoscopy room was not an enteric bacteria but a skin flora of healthcare staff that participated in endoscopic surgery. With regard to the endoscopy rooms where upper gastrointestinal endoscopy procedures were performed in the morning and colonoscopy, in the afternoon, as compared to the results of the environmental culture of samples collected in the afternoon those of samples collected in the morning showed a different distribution of bacterial isolates. This indicates that different skin floras of different examiners played an important role in the contamination of the environment. Given that most of the species belonged to skin flora, the differences in the distribution of bacterial isolates between the upper gastrointestinal endoscopy and colonoscopy procedures were not considered significant.
The study results showed that the species of bacterial isolates collected from the environment of the endoscopy room were non-pathogenic. However, such non-pathogenic bacteria can be the cause of severe illness in the case of immunocompromised patients who visit the endoscopy room.12,13 Therefore, more efforts need to be made to prevent the proliferation of bacteria by ensuring that doctors and nurses maintain a good practice of hand hygiene and by improving the overall environment inside the endoscopy room. Because the species of bacterial isolates collected in the study were not enteric bacteria but contaminants present on the skin of healthcare providers or the surrounding environment, the hand hygiene of healthcare staff is the most important factor in the management of the environment inside the endoscopy room. Although there was a possibility that bacterial contamination of the endoscope itself could be the cause of contamination within the endoscopy room, the 2 hospitals that participated in the study thoroughly cleaned endoscopes with neutral and enzyme detergents, sterilized and rinsed them with an automatic sterilizer, and kept them in the storage room after drying them using compressed air as well as properly sterilized the insertion parts and other accessories as well as the interior channel, in accordance with the quality management guidelines.14
So far, many studies have confirmed the presence of pathogens through environmental culture in the general wards and intensive care units. Hartmann et al. performed environmental culture on a doctor's computer keyboard and mouse, infusion pump, ventilators, a nurse's cart, the surface of telephones, all of which are commonly used in the intensive care unit. According to the results, Staphylococcus spp. accounted for 84.4% of all bacterial isolates, whereas pathogenic enterococcus and gram-negative bacteria accounted for around 3% each. In addition, Faires et al. performed environmental culture on a bulletin board, the backrest of a chair, the edge of beds, tables, curtains, televisions, etc., in a general ward to confirm the presence of methicillin-resistant S. aureus and C. difficile. Methicillin-resistant S. aureus and C. difficile accounted for 4% of all the bacterial isolates collected from the edges of beds and tables. Especially, the study classified the material of the surfaces into fabric, plywood, cork, and plastic and compared the culture results for all surfaces. The results showed that the plastic surface contained the highest CFUs, 6.8% of all the bacterial isolates.15
While this study confirmed the presence of pathogens through environmental culture in an endoscopy room, it had a limitation in terms of study subjects, as it targeted the endoscopy rooms of only 2 tertiary hospitals. Further studies involving several medical research centers and primary and secondary hospitals need to be conducted in the future. In addition, further studies could also identify a possible relation between the prevalence of hospital-acquired infections and presence of pathogens highlighted in this study.3
In conclusion, we did not find any pathogens in environmental culture of endoscopy units. However, because even a contaminant can result in immunocompromised patients getting infected, it is important to clean and disinfect the environment of an endoscopy room, with the hand hygiene of the healthcare staff being of utmost importance.
References
1. Trybou J, Spaepen E, Vermeulen B, Porrez L, Annemans L. Hospital-acquired infections in Belgian acute-care hospitals: financial burden of disease and potential cost savings. Acta Clin Belg. 2013; 68:199–205. PMID: 24156220.

2. Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999; 159:821–826. PMID: 10219927.

3. Hartmann B, Benson M, Junger A, et al. Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J Clin Monit Comput. 2004; 18:7–12. PMID: 15139578.

4. Santos LX, Souza Dias MB, Borrasca VL, et al. Improving hand hygiene adherence in an endoscopy unit. Endoscopy. 2013; 45:421–425. PMID: 23733725.

5. Gamboa F, Garcia DA, Acosta A, et al. Presence and antimicrobial profile of gram-negative facultative anaerobe rods in patients with chronic periodontitis and gingivitis. Acta Odontol Latinoam. 2013; 26:24–30. PMID: 24294820.
6. Dumford DM 3rd, Nerandzic MM, Eckstein BC, Donskey CJ. What is on that keyboard? Detecting hidden environmental reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains. Am J Infect Control. 2009; 37:15–19. PMID: 19171247.

7. Seo GS. Clostridium difficile Infection: What's New? Intest Res. 2013; 11:1–13.
8. Bashir MH, Olson LK, Walters SA. Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J Infect Control. 2012; 40:344–348. PMID: 21737178.

9. Borgaonkar MR, Hookey L, Hollingworth R, et al. Indicators of safety compromise in gastrointestinal endoscopy. Can J Gastroenterol. 2012; 26:71–78. PMID: 22312605.

10. Appelgate D, Faust B, Dunson J. Cleaning audits lead to better environmental hygiene. OR Manager. 2013; 29:110–11.
11. Matouskova I, Raida L, Holy O. The incidence of nonfermentative gram-negative bacilli in the environment of the transplant unit, department of hemato-oncology, university hospital Olomouc. Epidemiol Mikrobiol Imunol. 2012; 61:110–115. PMID: 23301626.
12. Drougka E, Foka A, Marangos MN, et al. The first case of Staphylococcus aureus ST398 causing bacteremia in an immunocompromised patient in Greece. Indian J Med Microbiol. 2012; 30:232–236. PMID: 22664446.

13. Dwyer DM, Klein EG, Istre GR, Robinson MG, Neumann DA, McCoy GA. Salmonella newport infections transmitted by fiberoptic colonoscopy. Gastrointest Endosc. 1987; 33:84–87. PMID: 3569805.

14. Sabnis RB, Bhattu A, Vijaykumar M. Sterilization of endoscopic instruments. Curr Opin Urol. 2014; 24:195–202. PMID: 24451088.

15. Faires MC, Pearl DL, Berke O, Reid-Smith RJ, Weese JS. The identification and epidemiology of meticillin-resistant Staphylococcus aureus and Clostridium difficile in patient rooms and the ward environment. BMC Infect Dis. 2013; 13:342. PMID: 23883171.
Fig. 1
The methods of environmental culture. (A) Sterile cotton-tipped swab moistened with sterile saline was used to swab the surfaces of 197 samples. (B) We cultured the swab in the blood agar plate. (C) Samples from the colonoscopy room were put in the thioglycollate broth. (D) The samples were cultured for 2 weeks.

Fig. 2
Distribution of microbial species isolated by culture of samples from various surfaces. BAC, Bacillus spp.; MIC, Micrococcus spp.; STR, Streptococcus spp.; STA, staphylococcus spp.; ETC, enterococcus.
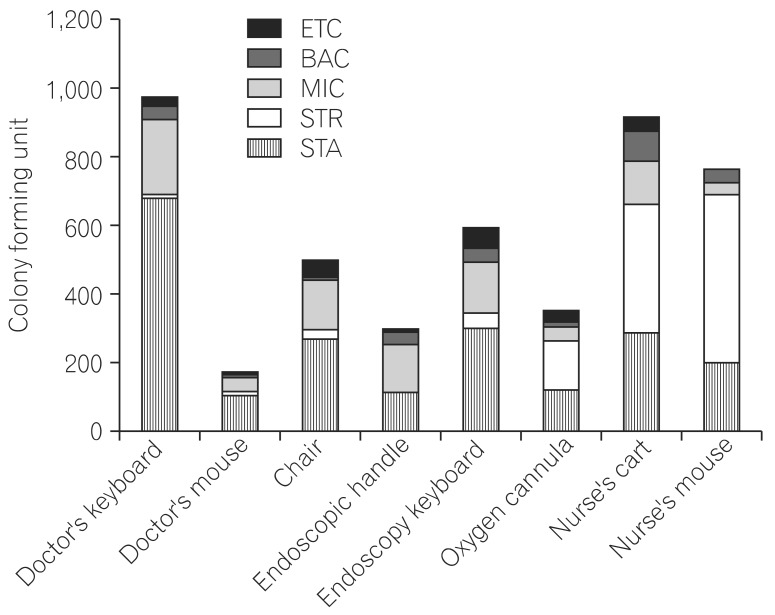
Fig. 3
Distribution of microbial species isolated by culture of samples from various surfaces. (A) Species of cultured microorganisms in the therapeutic endoscopy room. (B) Species of cultured microorganisms in the diagnostic upper endoscopy room. (C) Species of cultured microorganisms in the diagnostic colonoscopy room. BAC, Bacillus spp.; MIC, Micrococcus spp.; STR, Streptococcus spp.; STA, staphylococcus spp.; ETC, enterococcus.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download