Abstract
Background/Aims
A diagnosis of acute diverticulitis is based on computed tomography (CT). Colonoscopy is commonly performed after the acute event to exclude other diagnoses. This study aimed to determine whether colonoscopy is necessary and what additional information is gained from a colonoscopy after acute diverticulitis.
Methods
Acute diverticulitis was diagnosed by clinical criteria and characteristic CT findings. We analyzed the number of patients in whom colorectal cancers were diagnosed and other incidental findings of polyps and other diseases.
Results
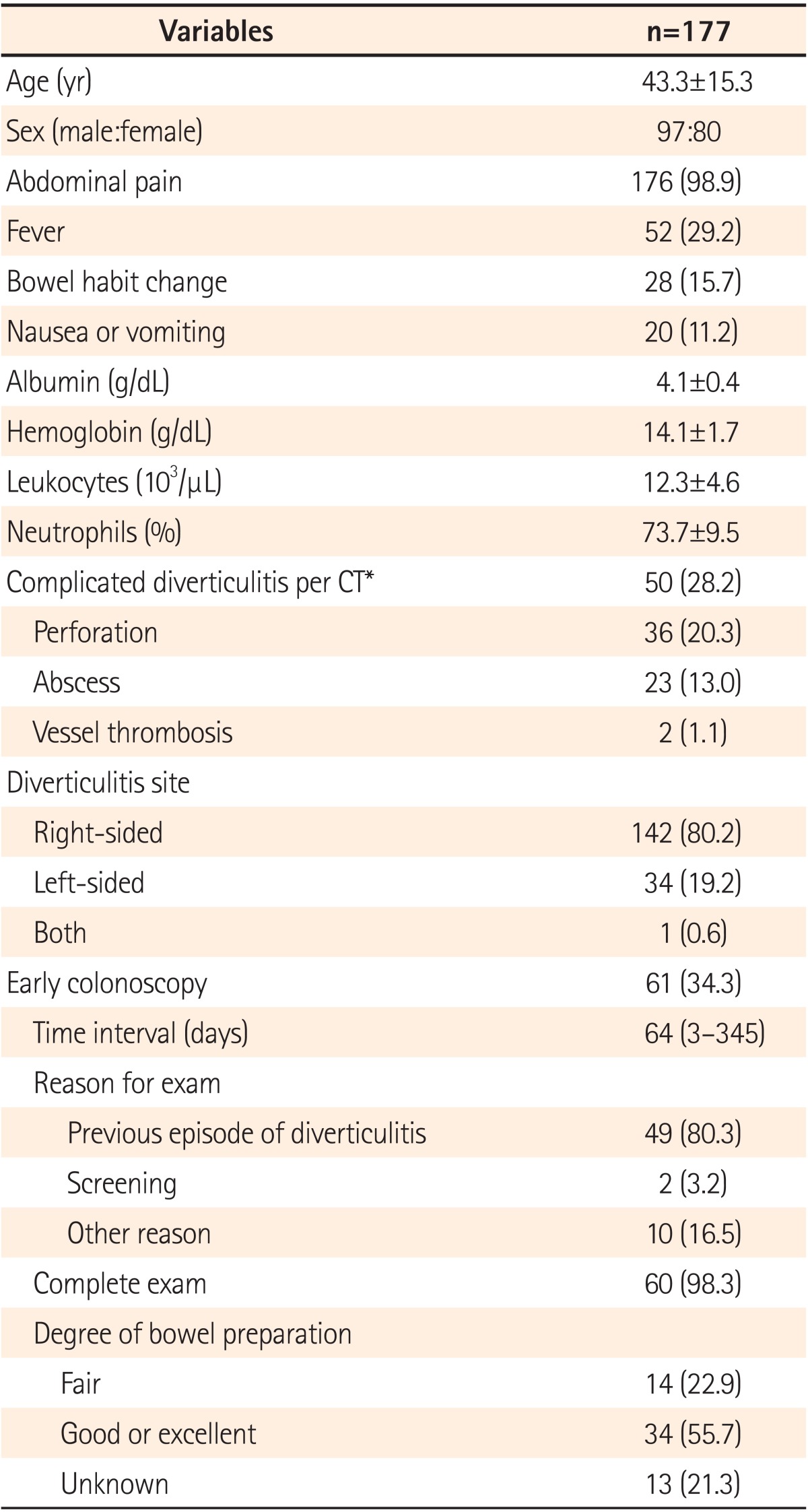
A total of 177 patients were analyzed retrospectively. The mean age was 43.3±15.3 years (range, 13-82 years) and 97 patients (54.8%) were male. Sixty-one patients had undergone a colonoscopy within 1 year of the acute attack. Advanced adenomatous lesions and colonic malignancy were not detected. Nineteen patients (31.1%) had ≥1 polyp and 11 patients (18.0%) had an adenomatous polyp. No new or different diagnosis was made after colonoscopy. None of the 116 patients who did not undergo colonoscopy within a year after acute diverticulitis had a diagnosis of colorectal cancer registered with the Korea Central Cancer Registry.
Acute colonic diverticulitis is an inflammatory process that complicates diverticular disease. The diagnosis of acute colonic diverticulitis is based on the clinical presentation and CT findings.1,2 CT enables an accurate diagnosis of diverticulitis, and objective classification into complicated and uncomplicated disease.3 Previous studies have investigated the accuracy of CT scans in diagnosing diverticulitis.4,5,6 However, the standard practice has been to use colonoscopy to confirm the presence of diverticular disease and to differentiate diverticulitis from other inflammatory and infectious conditions or colorectal cancer (CRC) in patients with a clinical diagnosis of diverticulitis after the inflammatory symptoms have completely resolved.7,8,9 This practice dates back to the time before the widespread use of CT to diagnose acute diverticulitis, and reflects the limitations of barium enema in differentiating between diverticular disease and other diseases. Moreover, a possible association between diverticulosis and CRC has been reported by several investigators.10,11,12,13 However, several recent studies failed to show any association or describe a decreased risk for CRC.14,15,16 There have been several reports about the usefulness of colonoscopy after acute diverticulitis.17,18,19 However, to our knowledge, no study has involved Asian populations. In regard to diverticular disease, the location and clinical manifestations were considerably different between Asian and other populations.20
This study aimed to assess the outcome of routine colonoscopy following acute diverticulitis, and what additional information can be gained from colonoscopy, particularly in excluding other diseases and in confirming the diagnosis.
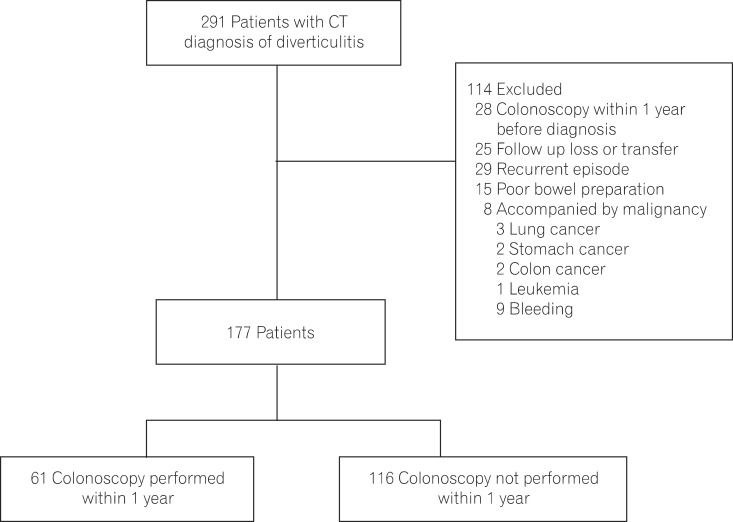
This was a retrospective, single center study. The medical records of 291 patients with a diagnosis of acute diverticulitis suggested by CT scan between November 1994 and December 2011 were reviewed. Patients were identified by the use of the picture archiving and communication system (PACS) database-a common statewide radiology database-and/or medical records using the reference term "diverticulitis". All CT examinations were performed with a helical CT with IV contrast enhancement (2.5-mm collimation and 2.5-mm intervals, HiSpeed Advantage; General Electric Medical Systems, Milwaukee, WI, USA). CT scans were interpreted by abdominal radiologists who have >5 years of experience. The CT criteria for acute diverticulitis included the presence of colonic inflamed diverticula (enhancement of thickened diverticular wall surrounded by the area of peridiverticular inflammation) with colonic wall thickening (wall thickness >3 mm on the short axis of the lumen) and/or surrounding fat stranding. The wall thickness was measured at the maximal magnification on a 2K×2K PACS monitor (General Electric Medical Systems Integrated Imaging Solutions, Mt. Prospect, IL, USA). CT findings in complicated diverticulitis included the presence of pericolic or abdominal abscess, localized or free extraluminal gas or contrast.21,22
Inclusion in this study was limited to patients with a CT diagnosis of acute diverticulitis without radiological features suspicious for colorectal neoplasia. For patients with recurrent presentations, only the first episode was included. If an individual had undergone >1 colonoscopy, only the study performed closest to the date of the diagnostic CT was included.
Patients presenting with diverticular bleeding were excluded, because the role and timing of a colonoscopy in the management of acute diverticular hemorrhage is controversial.23,24,25 Furthermore, we excluded patients with a past history or concomitant presence of malignancy, and colonoscopic reports with poor bowel preparation which was assessed using Aronchick's criteria.26 Patients who had undergone colonoscopy in the year prior to the current episode of acute diverticulitis were also excluded.
The clinical parameters, laboratory results, CT findings, colonoscopic findings of polyps, cancer or other non-neoplastic disease and histopathological reports were recorded. For patients who had not undergone colonoscopic exam in the year after acute diverticulitis, the Korea Central Cancer Registry was searched to identify malignancy cases. All outcomes were examined until 31 October 2012.
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Samsung Medical Center, Seoul, Republic of Korea (IRB No. 2013-10-039-001). Written informed consent was not required because this was a retrospective study.
Patients who had follow-up colonoscopy within 1 year from the date of CT scan were assigned to the early colonoscopy group (Group 1), to prevent the inclusion of patients who may have developed interval cancers after their diagnosis of diverticulitis. On the other hand, patients who had not undergone colonoscopy or had colonoscopy after 1 year from the date of CT scan were assigned to Group 2.
Histologic findings from the endoscopic biopsy of polyps were categorized on the basis of the most advanced lesion identified. Advanced adenomatous lesions were defined as either polyps ≥10 mm in diameter, and/or villous architecture and/or adenomas with high-grade dysplasia. Non-advanced adenomas were classified as <10 mm in diameter with low-grade dysplasia and <25% villous components. Hyperplastic polyps and non-neoplastic mucosal changes (for example, inflammatory polyp) were also recorded.
Data analysis was carried out using PASW version 19.0 (SPSS Inc., Chicago, IL, USA). For continuous variables such as age and blood chemistry measurements, descriptive statistics were calculated and reported as mean±SD and median. The normality of the distribution of the continuous variables was assessed using the Shapiro-Wilk test (cutoff at P=0.01). Categorical variables such as sex were described as frequency distributions and presented as frequencies (%). The t-test for independent samples or the Mann-Whitney U test was used to compare continuous variables between the subjects who underwent colonoscopy with those who did not. Categorical variables were compared by colonoscopy using the χ2 test or Fisher's exact test, as appropriate. All tests were considered significant at P<0.05.
Between November 1994 and December 2011, 914 patients with diverticular disease were initially identified by retrospective chart review. A radiological diagnosis of acute diverticulitis was made in 291 patients using the CT examination performed based on the clinical symptoms/signs. Finally, 177 patients were included in the analysis after application of the exclusion criteria (Fig. 1). Patient characteristics are summarized in Table 1. The mean age of the patients was 43.3±15.3 years (range, 13-82 years) and 97 patients (54.8%) were male.
Among the 177 cases of diverticulitis, 142 (80.2%) were predominantly right-sided, 34 (19.2%) were left-sided and one (0.6%) had involvement of both sites. In 163 of the 177 cases (92.1%) of diverticulitis, diverticulosis was noted on CT studies. However, in 14 of the 177 patients (7.9%), CT showed several findings suggesting diverticulitis such as colonic wall thickening >3 mm without diverticulosis (Fig. 2). Complications associated with diverticulitis were observed in 50 cases (28.2%) including perforation (n=36, 20.3%), abscess (n=23, 13.0%) and abscess combined with perforation (n=9, 5.1%). Twenty-nine (16.4%) patients in the cohort had undergone a surgical procedure. Twenty-four (13.5%) patients had an operation for intestinal perforation and/or abscess. Four (2.3%) patients complained of persistent symptoms despite antibiotic therapy. One (0.6%) patient underwent surgery to rule out malignancy; the final pathologic report showed no evidence of malignancy. Colonoscopy was performed in 4 of the 29 patients who underwent surgery. Only one of the patients-a man aged 55 years at the time of diagnosis-had a colonoscopy before surgical treatment. Although the colonoscopy findings were negative, the patient underwent a Hartmann's operation for a perforation complicated with diverticulitis 4 months later.
Sixty-one (34.3%) patients underwent colonoscopy in the first year after an acute diverticulitis episode (Fig. 2), for the following reasons: (1) routine exam following an acute diverticulitis event (n=49, 80.3%); (2) screening procedure (n=2, 3.2%); and (3) other reasons (n=10, 16.5%). The median time between the procedure and acute diverticulitis episode was 64 days (range, 3-345).
Most of the 48 colonoscopies (78.6%) had an excellent, good or fair bowel preparation, with the outcome in 13 of the colonoscopies being unknown. Complete colonoscopy with cecal intubation was achieved in 60 (98.3%) patients. One patient failed cecal intubation owing to a redundant colon. Complications such as bleeding or perforation did not occur during the colonoscopies. No significant differences were found in patient characteristics between the early colonoscopy and late or no-colonoscopy groups (Table 2).
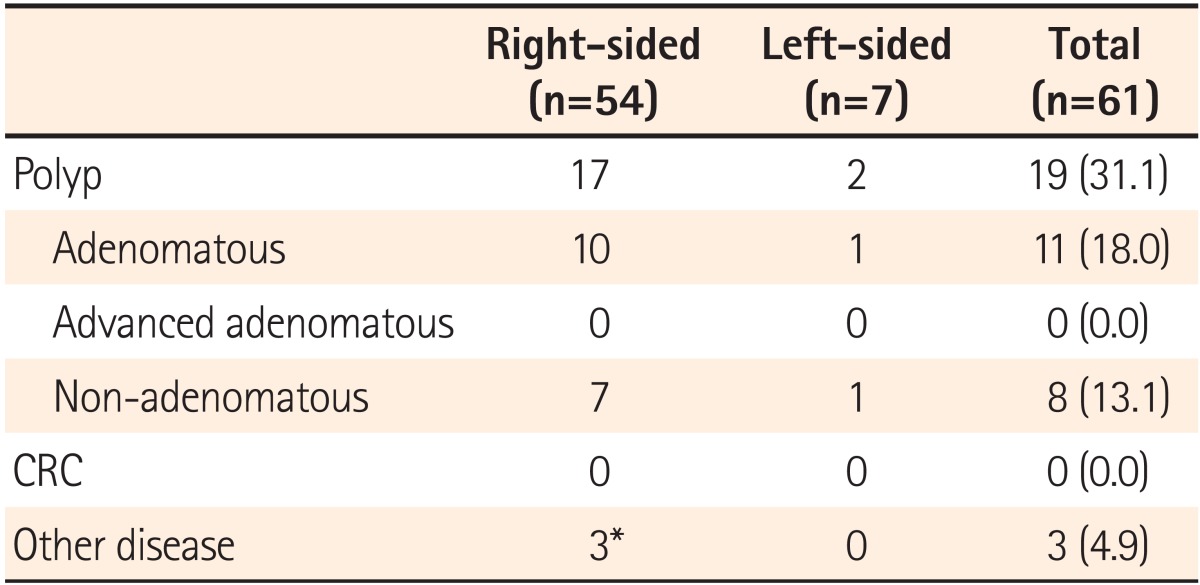
Polyps were detected in 19 patients (31.1%) and were removed by cold biopsy or snare in 17 patients (27.9%). Eleven patients (18.0%) had ≥1 adenomatous polyp. Moreover, no statistically significant difference in the polyp and adenoma detection rate was found in patients aged <50 years compared with older patients. Colon polyps were found in 11 (26.8%) of the 41 patients aged <50 years compared with 8 (40.0%) of the 20 patients aged ≥50 years (P=0.380). Seven (17.1%) of the 41 patients aged <50 years had adenomas compared with 4 (20.0%) of the 20 older patients (P=0.999). Advanced adenoma or carcinoma was not detected, and new or different diagnoses were not found. Only clinically insignificant diseases were found in 3 cases (4.9%). One case was a suspected appendiceal mucocele; however, the pathological findings showed no pathological alteration. The second case involved 2 small ulcers on the terminal ileum; the tissue was confirmed as an erosion, presumably due to a nonspecific ileal ulcer. The third case was a 1.2 cm lipoma on the ileo-caecal valve (Table 3).
Of the 61 patients undergoing colonoscopy, the CT findings suggested that 54 and 7 patients had right-sided and left-sided diverticulitis, respectively. With respect to the right-sided and left-sided diverticulitis, polyps were found in 17 and 2 cases (P=0.876), hyperplastic polyps in 6 and adenoma in 10 and 1 cases (P=0.999) and others (inflammatory polyps, etc.) in 4 and 1 cases, respectively. There were no significant differences according to the site of diverticulitis.
Of the 116 patients who did not undergo colonic evaluation, 3 died from other causes such as recurred ureteral cancer with bone metastasis, sudden arrest suspicious of pulmonary thromboembolism, and multi-organ failure from sepsis during postoperative intensive care after surgery for perforation as a complication of sigmoid colonic diverticulitis. Only one 50-year-old female patient had a rectal carcinoid tumor, found via colonoscopy 5 years from the time of diagnosis of diverticulitis, for which she underwent endosocopic resection.
None of these 116 patients had a diagnosis of malignancy, including CRC, registered with the Korea Central Cancer Registry at a median of 66 (range 11-202) months after the diverticulitis episode. Therefore, of the 177 patients with acute diverticulitis, no patients were diagnosed with CRC during follow-up.
Although CT is currently the most appropriate diagnostic imaging tool for diverticulitis, colonoscopy is commonly performed after an acute diverticulitis episode to exclude other infectious, inflammatory, or neoplastic diseases.27 In fact, the practice of performing colonoscopy after presumed diverticulitis to exclude colon cancer appears to have in large part arisen from concerns that colon cancer may be simulating the appearance of diverticulitis at CT.1 The isolated finding of colonic wall thickening appears to be associated with colon cancer, which may be a mimicker of diverticulitis.28,29 Although colonic wall thickening by itself is nonspecific, many authors have validated findings that accurately point to a diagnosis of diverticulitis, including inflamed diverticula, pericolic fat stranding, fluid at mesentery, and a preserved bowel enhancement pattern.30,31,32 Using these criteria, the overall accuracy of CT for the diagnosis of acute diverticulitis has been shown to be approximately 99%.6,31
In our study, the interobserver agreement was not assessed. However, the final CT findings were reported after a consensus was reached between the 2 radiologists.
A possible association between diverticulosis and CRC may rationalize the use of colonoscopy after acute diverticulitis, although it is controversial.10,11,13,15,20 Colonoscopy can cause rare, but serious, complications,33 and a higher failure rate of cecal intubation is associated with diverticular disease.19 Although the benefits of age-appropriate screening using colonoscopy cannot be overemphasized, the risks and costs of performing the procedure in all patients with diverticulitis are often overlooked. Recent studies to determine the yield of colonoscopy in diverticulitis cases suggested that routine colonoscopy after acute diverticulitis-diagnosed by typical clinical symptoms and CT findings-needs to be reevaluated.19,34,35,36 The findings of a prospective study19 suggested that colonoscopy performed immediately after an acute diverticulitis episode adds no clinical value; this result may be due to improved CT scanning. Another study, published before the advent of multidetector CT, reported the usefulness of colonoscopy after acute diverticulitis.37
The differentiation between diverticulitis and colon cancer by CT was difficult in several cases that have not been included in this study. An 81-year-old woman and a 61-year-old woman underwent CT examinations to differentiate between benign diverticular disease and malignancy, prior to undergoing colonoscopy-which enabled the diagnosis of colon cancer in the descending colon and hepatic flexure, respectively.
There are limited data about follow-up colonoscopy after CT diagnosis of acute diverticulitis. To our knowledge, there have been no studies concerning the usefulness of colonoscopy after acute diverticulitis in Asian populations. Diverticulosis in Asian populations presents several distinct characteristics compared to Western populations, predominantly being in the right-sided colon in relatively young patients with low complication rates.38,39,40,41 These findings are consistent with our study results, namely that right-sided diverticulitis was predominant (80.2%).
One of the possible explanations for the association between diverticular disease and CRC is that the presence of an inflammatory process increases the risk for malignant change, since both diseases occur predominantly in the left colon in Western populations.42 However, this hypothesis is not tenable in Asian populations, due to the right-sided nature of diverticulitis in the colon. It was found that the pathogenesis of colon cancer is different in left-sided and right-sided tumors.43 In this respect, further studies are necessary to evaluate the role of colonoscopy after acute diverticulitis in Asian populations.
In our study, polyps were found in 31.1% of patients and adenomatous polyps were evident in 18.0% of those who underwent colonoscopy within 1 year following an acute diverticulitis episode; these results are not higher than the polyp detection rate in an average-risk population. In a recent prospective multicenter study involving 2,307 adults aged ≥50 years who underwent screening using colonoscopy during 2003 or 2004, colorectal adenomas and advanced adenomas were found in 40.5% and 2.5% of Korean patients, respectively.44 According to a prospective study of colonoscopy screenings conducted in 11 Asian countries, the prevalence rates of colorectal neoplasia and advanced neoplasia in individuals aged >50 years were 23.9% and 5.8%, respectively. Moreover, no new or different diagnoses were made after colonoscopy in our study. As the incidence of polyps-including adenoma or colorectal neoplasia-increases with age, selecting older patients for colonoscopy following diverticulitis might be expected to improve the yield. However, this study found no statistically significant difference in polyp or adenoma detection rates in patients aged <50 years compared with older patients.
One-hundred-forty-two (80.0%) of the 177 cases of diverticulitis in our study were predominantly right-sided, whereas in Western populations diverticulitis is commonly limited to the sigmoid colon. There are several differences in the CT evaluation of the ascending and sigmoid colon. The sigmoid colon usually runs parallel to the axial scan, whereas the right-sided colon is perpendicular to the axial scan. Appendicitis and diverticulitis of the right colon are observed in younger populations than sigmoid diverticulitis; moreover, they were the most common differential diagnosis in our cases (11 cases, 6.1%). It was difficult to differentiate appendicitis from right diverticulitis in the case of pericecal inflammatory stranding in the absence of a visualized appendix. In 3 cases (1.7%), colitis-such as intestinal tuberculosis and IBD-had to be ruled out at CT, unexpectedly. For these diseases, the degree of colonic wall thickening typically exceeds the degree of associated fat stranding; however, in diverticulitis, fat stranding is more severe than expected for the degree of bowel wall thickening present. In this regard, infectious diseases involving the right-sided colon-such as IBD or intestinal tuberculosis-need to be carefully considered as a differential diagnosis of diverticulitis in Asian patients.
Our study has several strengths. First, to our knowledge, our study is the first to present data in an Asian cohort. Second, we have investigated the long-term follow-up outcomes of patients whose CT report was strongly suggestive of acute diverticulitis; this is significant because the prevalence of diverticulitis is expected to increase in Asia due to Westernization of life style and diet.38,45
The limitations of this study include the limited follow-up colonoscopy reports. It is likely that the prevalence of adenoma or colon cancer in patients suspected of having diverticulitis was underestimated. Nevertheless, we followed the patients who underwent colonoscopy >1 year from the diagnosis of diverticulitis or those who did not undergo colonoscopy, with a long follow-up period (median of 66 months) with the Korea Central Cancer Registry; during follow-up, no patient had a diagnosis of malignancy, including CRC. Second, it is difficult to determine the comparison group from the general population for the analysis of polyp and CRC detection rate. Moreover, the demographic characteristics were not compared and confounding factors such as family history of CRC were not considered.
In conclusion, routine colonoscopy yields little benefit in patients with acute diverticulitis and more refined criteria should be developed. The use of colonoscopy should be limited to situations in which the diagnosis of diverticulitis is unclear. In the absence of other indications, a subsequent colonoscopic evaluation may not be required to confirm the diagnosis. A large prospective study is necessary to assess the validity of the recommendation for colonoscopic follow-up after a CT diagnosis of acute diverticulitis.
Notes
References
1. Cho KC, Morehouse HT, Alterman DD, Thornhill BA. Sigmoid diverticulitis: diagnostic role of CT--comparison with barium enema studies. Radiology. 1990; 176:111–115. PMID: 2191360.

2. Rao PM. CT of diverticulitis and alternative conditions. Semin Ultrasound CT MR. 1999; 20:86–93. PMID: 10222517.

3. Ambrosetti P, Jenny A, Becker C, Terrier TF, Morel P. Acute left colonic diverticulitis--compared performance of computed tomography and water-soluble contrast enema: prospective evaluation of 420 patients. Dis Colon Rectum. 2000; 43:1363–1367. PMID: 11052512.

4. Lameris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008; 18:2498–2511. PMID: 18523784.

5. Pradel JA, Adell JF, Taourel P, Djafari M, Monnin-Delhom E, Bruel JM. Acute colonic diverticulitis: prospective comparative evaluation with US and CT. Radiology. 1997; 205:503–512. PMID: 9356636.

6. Rao PM, Rhea JT, Novelline RA, et al. Helical CT with only colonic contrast material for diagnosing diverticulitis: prospective evaluation of 150 patients. AJR Am J Roentgenol. 1998; 170:1445–1449. PMID: 9609151.
7. Ben Yaacoub I, Boulay-Coletta I, Julles MC, Zins M. CT findings of misleading features of colonic diverticulitis. Insights Imaging. 2011; 2:69–84. PMID: 22347935.
8. Rafferty J, Shellito P, Hyman NH, Buie WD. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006; 49:939–944. PMID: 16741596.
9. Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med. 2007; 357:2057–2066. PMID: 18003962.
10. Choi CS, Choi SC, Seo GS, et al. Association between diverticulosis and colonic neoplasm in Koreans. Korean J Gastroenterol. 2007; 49:364–368. PMID: 17641554.
11. Kieff BJ, Eckert GJ, Imperiale TF. Is diverticulosis associated with colorectal neoplasia? A cross-sectional colonoscopic study. Am J Gastroenterol. 2004; 99:2007–2011. PMID: 15447764.

12. Stefansson T, Ekbom A, Sparen P, Pahlman L. Increased risk of left sided colon cancer in patients with diverticular disease. Gut. 1993; 34:499–502. PMID: 8491397.

13. Morini S, Hassan C, Zullo A, et al. Diverticular disease as a risk factor for sigmoid colon adenomas. Dig Liver Dis. 2002; 34:635–639. PMID: 12405250.

14. Krones CJ, Klinge U, Butz N, et al. The rare epidemiologic coincidence of diverticular disease and advanced colonic neoplasia. Int J Colorectal Dis. 2006; 21:18–24. PMID: 15889263.

15. Lam TJ, Meurs-Szojda MM, Gundlach L, et al. There is no increased risk for colorectal cancer and adenomas in patients with diverticulitis: a retrospective longitudinal study. Colorectal Dis. 2010; 12:1122–1126. PMID: 19575738.

16. Meurs-Szojda MM, Terhaar sive Droste JS, Kuik DJ, Mulder CJ, Felt-Bersma RJ. Diverticulosis and diverticulitis form no risk for polyps and colorectal neoplasia in 4,241 colonoscopies. Int J Colorectal Dis. 2008; 23:979–984. PMID: 18594842.

17. Sakhnini E, Lahat A, Melzer E, et al. Early colonoscopy in patients with acute diverticulitis: results of a prospective pilot study. Endoscopy. 2004; 36:504–507. PMID: 15202046.

18. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011; 54:1265–1270. PMID: 21904141.

19. Lahat A, Yanai H, Menachem Y, Avidan B, Bar-Meir S. The feasibility and risk of early colonoscopy in acute diverticulitis: a prospective controlled study. Endoscopy. 2007; 39:521–524. PMID: 17554647.

20. Lee SJ, Kim SA, Ku BH, et al. Association between colorectal cancer and colonic diverticulosis: case-control study based on computed tomographic colonography. Abdom Imaging. 2012; 37:70–73. PMID: 21516446.

21. Hemming J, Floch M. Features and management of colonic diverticular disease. Curr Gastroenterol Rep. 2010; 12:399–407. PMID: 20694839.

22. Destigter KK, Keating DP. Imaging update: acute colonic diverticulitis. Clin Colon Rectal Surg. 2009; 22:147–155. PMID: 20676257.

23. Smoot RL, Gostout CJ, Rajan E, et al. Is early colonoscopy after admission for acute diverticular bleeding needed? Am J Gastroenterol. 2003; 98:1996–1999. PMID: 14499777.

24. Foutch PG, Zimmerman K. Diverticular bleeding and the pigmented protuberance (sentinel clot): clinical implications, histopathological correlation, and results of endoscopic intervention. Am J Gastroenterol. 1996; 91:2589–2593. PMID: 8946992.
25. Savides TJ, Jensen DM. Colonoscopic hemostasis for recurrent diverticular hemorrhage associated with a visible vessel: a report of three cases. Gastrointest Endosc. 1994; 40:70–73. PMID: 8163141.

26. Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000; 52:346–352. PMID: 10968848.

27. Bauer VP. Emergency management of diverticulitis. Clin Colon Rectal Surg. 2009; 22:161–168. PMID: 20676259.

28. Wolff JH, Rubin A, Potter JD, et al. Clinical significance of colonoscopic findings associated with colonic thickening on computed tomography: is colonoscopy warranted when thickening is detected? J Clin Gastroenterol. 2008; 42:472–475. PMID: 18344892.

29. Cai Q, Baumgarten DA, Affronti JP, Waring JP. Incidental findings of thickening luminal gastrointestinal organs on computed tomography: an absolute indication for endoscopy. Am J Gastroenterol. 2003; 98:1734–1737. PMID: 12907326.

30. Shen SH, Chen JD, Tiu CM, et al. Differentiating colonic diverticulitis from colon cancer: the value of computed tomography in the emergency setting. J Chin Med Assoc. 2005; 68:411–418. PMID: 16187597.

31. Jang HJ, Lim HK, Lee SJ, Lee WJ, Kim EY, Kim SH. Acute diverticulitis of the cecum and ascending colon: the value of thin-section helical CT findings in excluding colonic carcinoma. AJR Am J Roentgenol. 2000; 174:1397–1402. PMID: 10789802.
32. Chintapalli KN, Chopra S, Ghiatas AA, Esola CC, Fields SF, Dodd GD 3rd. Diverticulitis versus colon cancer: differentiation with helical CT findings. Radiology. 1999; 210:429–435. PMID: 10207426.

33. Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006; 145:880–886. PMID: 17179057.

34. Westwood DA, Eglinton TW, Frizelle FA. Routine colonoscopy following acute uncomplicated diverticulitis. Br J Surg. 2011; 98:1630–1634. PMID: 21713756.

35. Sai VF, Velayos F, Neuhaus J, Westphalen AC. Colonoscopy after CT diagnosis of diverticulitis to exclude colon cancer: a systematic literature review. Radiology. 2012; 263:383–390. PMID: 22517956.

36. Schmilovitz-Weiss H, Yalunin E, Boaz M, et al. Does a colonoscopy after acute diverticulitis affect its management?: a single center experience. J Clin Gastroenterol. 2012; 46:317–320. PMID: 22186742.
37. Boulos PB, Karamanolis DG, Salmon PR, Clark CG. Is colonoscopy necessary in diverticular disease? Lancet. 1984; 1:95–96. PMID: 6140435.

38. Takano M, Yamada K, Sato K. An analysis of the development of colonic diverticulosis in the Japanese. Dis Colon Rectum. 2005; 48:2111–2116. PMID: 16228844.

39. Simpson J, Neal KR, Scholefield JH, Spiller RC. Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol. 2003; 15:1005–1010. PMID: 12923374.

40. Kim HU, Kim YH, Choe WH, et al. Clinical characteristics of colonic diverticulitis in Koreans. Korean J Gastroenterol. 2003; 42:363–368. PMID: 14646572.
41. Lee SJ, Shin JE, Cho SY, et al. Clinical predictors associated with the severity of colonic diverticulitis. Intest Res. 2013; 11:23–27.

42. Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004; 291:585–590. PMID: 14762037.

43. Bauer KM, Hummon AB, Buechler S. Right-side and left-side colon cancer follow different pathways to relapse. Mol Carcinog. 2012; 51:411–421. PMID: 21656576.

44. Park HW, Byeon JS, Yang SK, et al. Colorectal neoplasm in asymptomatic average-risk Koreans: the KASID prospective multicenter colonoscopy survey. Gut Liver. 2009; 3:35–40. PMID: 20479899.

45. Song JH, Kim YS, Lee JH, Ok KS, Ryu SH, Moon JS. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med. 2010; 25:140–146. PMID: 20526386.

Fig. 2
Acute diverticulitis. (A) Diverticulitis of the ascending colon in a 34-year-old man. The axial CT scan shows marked wall thickening of the ascending colon with an inflamed diverticulum (long arrow) and pericolic infiltration (short arrow). (B) Diverticulitis of the ascending colon in a 40-year-old man. Colonoscopy shows a diverticular orifice (black arrow) appearing inflamed, with mucopurulent exudate on the ascending colon.
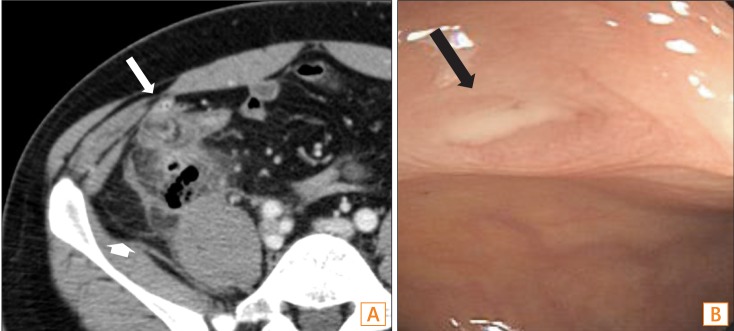
Table 2
Comparison between the Early Colonoscopy Group (Group 1) and Late or No-Colonoscopy Group (Group 2)
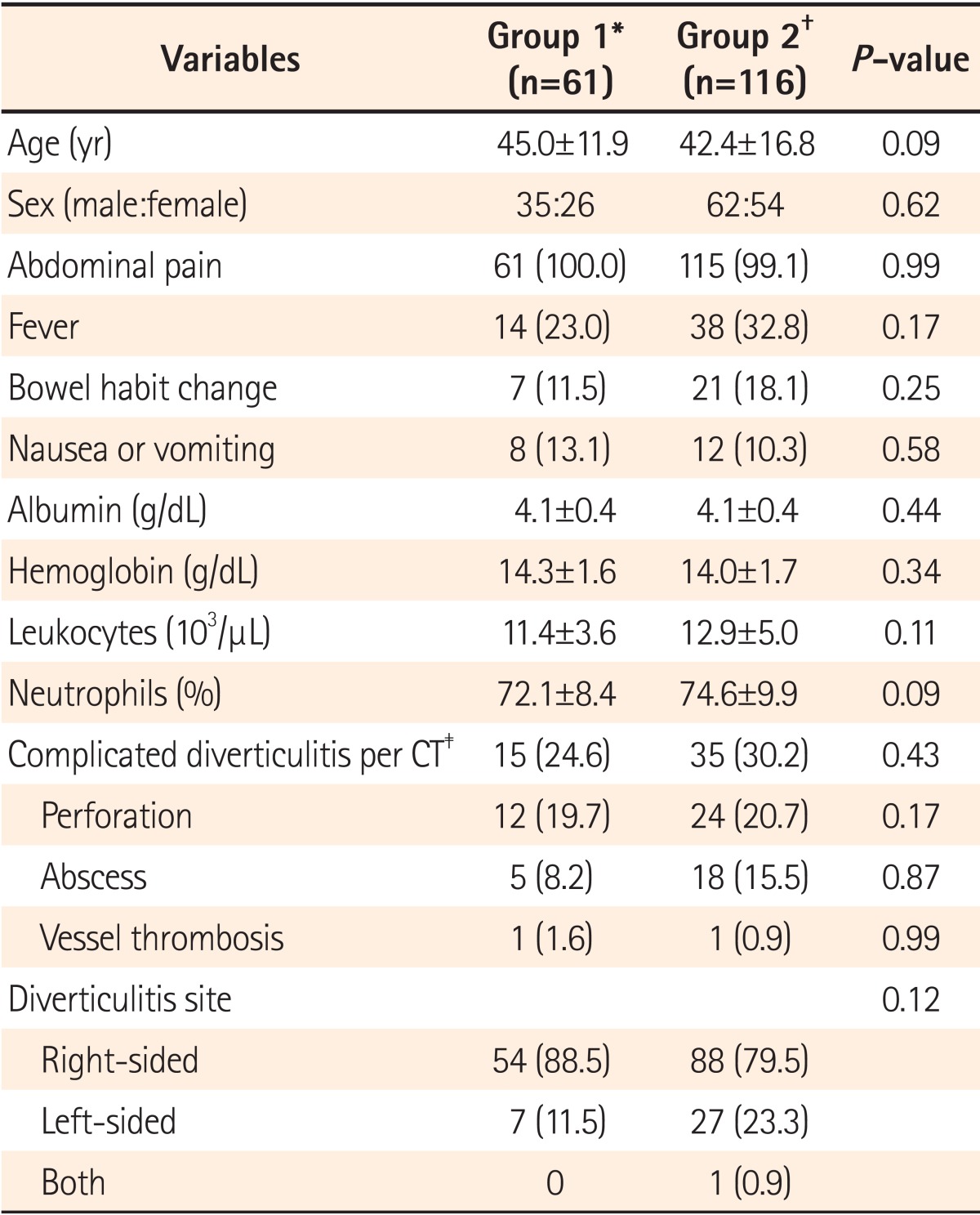
Values are presented as mean±SD or n (%).
*Group 1: Patients who underwent colonoscopy within 1 year after diagnosis of diverticulitis.
†Group 2: Patients who did not undergo colonoscopy within 1 year (did not undergo colonoscopy or underwent colonoscopy after >1 year from the diagnosis of diverticulitis).
‡Multiple choices.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download