Crohn's disease (CD) is a chronic, relapsing immune-mediated inflammatory disorder of the intestine with unknown causes. Both genetic and environmental factors are known to be involved in the pathogenesis of the disease.1 The incidence and prevalence of CD have recently been increasing in Korea, probably secondary to environmental changes.2 To address this challenge, we need to understand the specific clinical and therapeutic characteristics of CD in Korean patients. It has been widely accepted that the phenotypes of IBD differ considerably between East Asians and Caucasians.3,4 Thus, the management strategies used for patients with CD in Western countries may not be appropriate for those in Korea. Therefore, it is crucial to understand the natural history and clinical characteristics of this disease in Korean patients, which are largely unknown due to the lack of population-based cohort studies. Most of the currently available data comes from single-center, hospital-based studies. Several important cohort studies conducted in Western Europe and America have described the clinical course of CD.5,6,7 But there have been no well-designed cohort studies in Korea. Consequently, investigators are becoming aware of the need for a cohort study of CD in Korea. Moreover, genetic or biomarker studies need blood or tissue specimens from a large number of patients, which should be collected together with accurate, matched clinical data. This highlights the urgent need for a nationwide collection of well-defined clinical characteristics of Korean CD patients and their biological specimens.
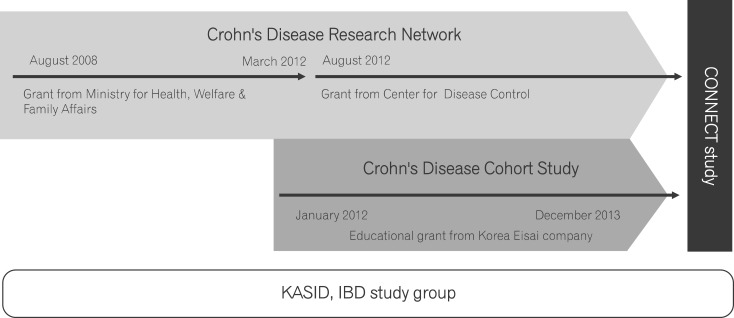
Attempts to comprehensively understand the epidemiologic, clinical, and genetic characteristics of Korean CD patients started in 2008. Won Ho Kim at Yonsei University, President of the Korean Association for the Study of Intestinal Diseases (KASID) at that time, obtained a national grant, the "Research Network for Crohn's Disease." The four major aims of the study were as follows: (1) understand the epidemiologic and clinical characteristics by establishing a registered database of CD patients; (2) establish a genetic research management system and a genetic biobank by obtaining blood specimens and clinical data from CD patients; (3) establish a network of doctors specialized in CD treatment and patients with CD as a private non-profit organization; and (4) develop specific Korean diagnostic and therapeutic guidelines for CD. During the study period, clinical data from nearly 2,000 blood specimens (both genomic DNA and sera) of 1,316 Korean CD patients were collected and stored at the Korean Green Cross Corporation. Active translational research by basic and clinical researchers is now underway using these samples.8,9 In addition, this study team established a nationwide doctor-patient network by hosting educational workshops for patients and symposiums for clinicians via the regional society meetings every year (Seoul, Gyunggi, Daegu, and Daejeon). These provided disease information, life style education, counseling, and various programs for patients and their families, including one ensuring patients' rights. This study team also established and published the Korean diagnostic and therapeutic guidelines for CD with the help of the IBD Study Group of KASID10,11 that published the diagnostic guidelines for intestinal tuberculosis, a disease often misdiagnosed as CD, in the same year.12 Through an e-mail survey, the IBD Study Group of KASID monitored the effectiveness of the diagnostic guidelines in clinical practice, investigated clinician satisfaction with the guidelines, and collected feedback on ways to improve the guidelines.13 At that time, the chair of IBD study group was Professor Joo Sung Kim at Seoul National University.
After the nationally funded Research Network for Crohn's Disease study ended, a better-designed cohort study involving prospective collection of clinical data was planned. The Korea Eisai Company sponsored a two-year educational grant for this CD cohort study. This study followed the Research Network for Crohn's Disease study and was named Establishment of Crohn's disease in Korea and characterization of clinical features with long-term follow-up, and was conducted from January 2012 to December 2013. This study team was led by Dong Soo Han, a Professor at the Hanyang University and the current vice president of KASID. Retrospective clinical data (for patients enrolled before 2009) and prospective clinical data (for patients enrolled during and after 2009) were collected in this study. The prospective patients were enrolled using a novel web-based eCRF system (www.cdcohort.org). Data for 1,388 retrospective patients and 890 prospective patients were collected and analyzed. A study coordinator monitored submission and quality of eCRF data. Blood specimens were collected from 635 of the prospective patients and stored in a deep freezer at -80℃. During this study, the IBD study group chair was Professor Young Ho Kim at the Sungkyunkwan University, who organized the clinical and translational studies that utilized these data. This study was registered at www.clinicaltrials.gov. The first results using the prospective data were presented during the 2014 regular meeting of the European Crohn's and Colitis Organization in Copenhagen, Denmark. The most important and innovative outcomes from this study were the establishment of a novel eCRF system to collect well-recorded, high-quality prospective data and the development of the associated data input protocol (the latter of which was led by Professor Byong Duk Ye).
In 2012, the Cardiovascular and Rare Disease Section, Korea Centers for Disease Control and Prevention (www.cdc.go.kr) solicited grant applications for a 6-month demonstration project aimed at establishing a CD network (to run from August 2012 to December 2012). The KASID obtained this grant, and in 2012, for a 3-year national grant from the Korea Centers for Disease Control and Prevention. The new study funded by these grants was named Establishment and Management of Crohn's Disease Research Network. The principal aim of this study was to broaden the pool of study subjects by recruiting additional investigators throughout Korea. Consequently, 28 institutions were recruited to participate in this study. Currently, Professor Joo Sung Kim at the Seoul National University is in charge of the study. The current chair of the IBD study group is Professor Kang Moon Lee at the Catholic University, and he is currently supervising the investigators and study projects working with the collected data.
Recently, the latter two studies discussed above were integrated into a single study referred to as the Crohn's Disease Clinical Network and Cohort (CONNECT) study (Fig. 1). A total of 34 institutions are now participating in this study, and 1,900 blood specimens have been collected. Four abstracts were presented at the regular KASID meeting in April 2014. From the CONNECT study, investigators anticipate that the typical clinical characteristics and genetic causes of CD in Korean patients will be elucidated. Additional diagnosis and treatment guidelines and therapeutic agents for Korean CD patients will be developed based on these data. Additionally, we anticipate that after analysis, the collected data will be presented at international symposiums and the papers published. By following up with the registered patients, long-term clinical characteristics and accuracy of initial diagnosis can also be investigated. Long-term follow-up results from the CONNECT study should help clinicians and researchers more accurately define CD prognosis in Korea, provide specific health care planning and education, and identify causative factors for CD in Korea, where the incidence of CD is rapidly increasing.
Notes
References
1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007; 448:427–434. PMID: 17653185.

2. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008; 103:3167–3182. PMID: 19086963.

3. Cheon JH. Genetics of inflammatory bowel diseases: a comparison between Western and Eastern perspectives. J Gastroenterol Hepatol. 2013; 28:220–226. PMID: 23189979.

4. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14. PMID: 20479907.

5. Solberg IC, Cvancarova M, Vatn MH, Moum B. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn's disease (the IBSEN Study). Inflamm Bowel Dis. 2014; 20:60–68. PMID: 24280875.

6. Clara I, Lix LM, Walker JR, et al. The Manitoba IBD Index: evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009; 104:1754–1763. PMID: 19455122.

7. Jess T, Loftus EV Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006; 130:1039–1046. PMID: 16618397.

8. Kim BJ, Choi YS, Jang BI, et al. Prospective evaluation of the clinical utility of interferon-gamma assay in the differential diagnosis of intestinal tuberculosis and Crohn's disease. Inflamm Bowel Dis. 2011; 17:1308–1313. PMID: 21053248.

9. Kim YS, Kim YH, Kim WH, et al. Diagnostic utility of anti-Saccharomyces cerevisiae antibody (ASCA) and Interferon-gamma assay in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Clin Chim Acta. 2011; 412:1527–1532. PMID: 21575618.

10. Ye BD, Yang SK, Shin SJ, et al. Guidelines for the management of Crohn's disease. Intest Res. 2012; 10:26–66.

11. Ye BD, Jang BI, Jeen YT, Lee KM, Kim JS, Yang SK. Diagnostic guideline of Crohn's disease. Korean J Gastroenterol. 2009; 53:161–176. PMID: 19835218.
12. Kim YS, Kim YH, Lee KM, Kim JS, Park YS. Diagnostic guideline of intestinal tuberculosis. Korean J Gastroenterol. 2009; 53:177–186. PMID: 19835219.
13. Park SJ, Cheon JH, Ye BD, et al. A survey of actual clinical application patterns in Korean diagnostic guidelines for inflammatory bowel disease. Korean J Gastroenterol. 2012; 60:292–299. PMID: 23172277.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download