Abstract
Angiogenesis is the formation of new blood vessels from existing ones and an underlying cause of numerous human diseases, including cancer and inflammation. A large body of evidence indicates that angiogenic inhibitors have therapeutic potential in the treatment of vascular diseases. However, detrimental side effects and low efficacy hinder their use in clinical practice. Members of the corticotropin-releasing hormone (CRH) family, which comprises CRH, urocortin I-III, and CRH receptors (CRHR) 1 and 2, are broadly expressed in the brain and peripheral tissues, including the intestine and cardiovascular system. The CRH family regulates stress-related responses through the hypothalamic-pituitary-adrenal axis. Therapeutic agents that target CRH family members offer a new approach to the treatment of various gastrointestinal disorders, including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and colorectal cancer. Since the discovery that CRHR 2 has anti-angiogenic activity during postnatal development in mice, studies have focused on the role of the CRH system in the modulation of blood vessel formation and cardiovascular function. This review will outline the basic biological functions of the CRH family members and the implications for the development of novel anti-angiogenic therapies.
Corticotropin-releasing hormone (CRH), first characterized by Vale et al. in 1981, is a neurohormonal activator of the hypothalamic-pituitary-adrenal (HPA) axis that stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, leading to the release of corticosteroids from the adrenal gland.1 Therefore, CRH is a major modulator of various stress-induced behavioral, autonomic, and visceral changes.
The CRH system regulates stress-related changes in the gastrointestinal tract. Consequently, it has been implicated as a therapeutic target for the treatment of IBS. Central administration of CRH and its related peptides, urocortin (Ucn) I-III, mimics the stress-induced colonic response as follows: (1) CRH, Ucn I, and Ucn II inhibit gastric emptying and suppress propagative contractions by activating CRH receptor 2 (CRHR2).2 This response is mediated by stimulation of the autonomic nervous system, not by the HPA axis.3 (2) CRH and Ucn I inhibit small intestinal transit and motility to a lesser extent than they inhibit gastric transit because autonomic control is less prominent in the small intestine than in the stomach.4,5 (3) CRH and Ucn I increase colonic motility by activating CRHR1 independent of HPA axis activation.2,3 In contrast, peripheral administration of CRH and Ucn I inhibits gastric emptying through the activation of CRHR2, and it delays small intestinal transit and increases colonic motility through CRHR1 activation.6
Cellular responses and signaling pathways induced by the CRH family may also affect intestinal inflammation. Activation of CRHR1 increased dextran sodium sulfate-induced colitis, whereas activation of CRHR2 decreased it.7 In addition, CRH and CRHR2 have been implicated as inflammatory inducers in Clostridium difficile toxin A-induced enteritis.8,9 In trinitrobenzenesulfonic acid-induced colitis, centrally administered CRH reduced inflammation.10 Inhibiting CRHR1 activity with a specific antagonist, antalarmin, suppressed endotoxin-induced inflammation, resulting in reduced inflammatory cytokine production.11
As a critical pathological component of gastrointestinal diseases, including IBD and colon cancer, angiogenesis facilitates disease progression by supplying essential nutrients and by promoting the recruitment of immune cells and the release of cytokines, chemokines, and matrix-degrading enzymes.12 Regulators of angiogenesis, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor , and interleukin-8, are potential targets for the treatment of numerous vascular diseases. Emerging evidence suggests that the CRH family is a novel angiogenic regulator in endogenous and inflammatory conditions. This review highlights the possible role of the CRH family in angiogenesis.
To date, four members of the CRH family have been identified, including CRH, Ucn I, Ucn II (stresscopin-related peptide), and Ucn III (stresscopin).13,14,15 CRH is a 41-amino acid peptide isolated from the ovine hypothalamus. Its primary structure is well conserved among mammalian species.16 Three novel CRH-related peptides were characterized more recently. Ucn I, a 40-amino acid peptide isolated from the rat midbrain, and two 38-amino acid peptides, Ucn II and III, show 45%-18% homology, respectively, with rat/human CRH.17
CRH-deficient mice exhibited normal postnatal growth, fertility, and longevity despite marked glucocorticoid deficiency.18 Fetal development, however, was profoundly affected by this deficiency; the progeny of homozygous CRH-deficient mice all died within the first 12 h after birth as a result of lung dysplasia, including marked hypercellularity, thickened alveolar septae, and a paucity of air spaces. In addition, although the pituitary gland appeared normal, marked atrophy of the zona fasciculata of the adrenal gland, the area primarily responsible for corticosterone production in mice, was observed. The adrenal stress response was impaired and sexually dimorphic, resulting in reduced production of corticosterone in male mice. These observations suggest that CRH plays a critical role during stress.
Urocortin-deficient mice exhibited increased anxiety-like behaviors in the elevated plus maze and open-field tests, but their acute restraint-induced stress responses did not differ from those in wild-type controls, suggesting that endogenous Ucn is not involved in the HPA axis-mediated stress response.19 The increased anxiety-like behavior of Ucn-null mice was not due to altered expression of CRH or CRHR1, but the levels of CRHR2 mRNA in the lateral septum were significantly reduced, suggesting that Ucn modulates anxiety through CRHR2. Although central administration of Ucn induced a stress response and suppressed food intake, deficiency in endogenous Ucn did not alter the basal feeding behavior. Furthermore, Ucn is expressed in central auditory pathways, where it affects auditory function. Consequently, hearing was impaired in Ucn-deficient mice.19,20
CRH ligands bind to two highly homologous receptors, CRHR1 and CRHR2, which belong to the G-protein-coupled receptor family (Fig. 1).21 CRHR1 and CRHR2 bind differentially to each of the CRH family members. CRHR1 shows high affinity for CRH and Ucn I, but no appreciable affinity for Ucn II and Ucn III. CRHR2 binds with greater affinity to Ucn I, Ucn II, and Ucn III than to CRH. Extensive alternate splicing of CRHR1 and CRHR2 produces various isoforms: CRHR1α, CRHR2α, CRHR2β, and CRHR2γ.22 In general, CRHR1 mediates the "fight-or-flight" stress response through the CRH-ACTH-glucocorticoid axis, whereas CRHR2 mediates stress-coping responses through Ucn II (stresscopin) and Ucn III (stresscopin-related peptide).23
CRHR1-deficient mice exhibited a marked decrease in the size of the zona fasciculata region of the adrenal gland and thus had a low plasma concentration of corticosterone.24 Although basal ACTH secretion was normal in these mice, restraint stress did not increase circulating ACTH levels. The progeny born to homozygous mutant females developed lung dysplasia with alveolar collapse and emphysema and died within 2 days after birth. In utero corticosterone treatment rescued the phenotype. The mutant mice were less sensitive to anxiogenic-like stimuli, suggesting that CRHR1 is required for a normal anxiety response.
CRHR2-deficient mice showed increased anxiety-like behavior, and they were hypersensitive to the HPA axis-mediated stress response.25 Although the food intake pattern and weight changes were normal, the mice exhibited increased anxiety, decreased appetite, and thus reduced food intake in response to food deprivation stress. Intravenous infusion of Ucn did not reduce the mean arterial pressure in the mutant mice, but the nitric oxide-mediated vasodilation response in the peripheral vasculature was normal.
Thus, these findings suggest that CRHR1 mutant mice show decreased anxiety-like behavior, and CRHR2 mutant mice show increased anxiety-like behavior.
The biological activities of the CRH family are modulated by CRH-binding protein (CRH-BP) and a soluble splice variant of CRHR2α (sCRHR2α).26,27 CRH-BP is a secreted glycoprotein that binds to CRH and Ucn I. sCRHR2α also binds to CRH and Ucn I and reduces their biological activity. Neither Ucn II nor Ucn III exhibits appreciable affinity for CRH-BP or sCRHR2α.
A large body of evidence supports the idea that the CRH system regulates angiogenesis. The endogenous role of the CRH system in vascular development was first examined by Bale et al.28 CRHR2 inhibited postnatal neovascularization, as evidenced by hypervascularization in CRHR2-deficient mice by postnatal day 21. The underlying mechanisms included inhibition of smooth muscle cell (SMC) proliferation, reduction of VEGF release from SMCs, and inhibition of in vitro angiogenesis in endothelial cells. Interestingly, the increased vascularity in CRHR2-deficient mice involved both capillaries and large vessels. Furthermore, hypervascularization did not occur during development but progressed from birth into adulthood. This finding suggests that CRH is a potential therapeutic target for vascular disease intervention, including the treatment of cancer and chronic inflammatory diseases.
CRHR2 could affect vascular development through two mechanisms: nitric oxide (NO) production and hypoxia.29 NO increases endothelial cell survival and thus regulates angiogenesis.30 In addition, inhibition of NO production may increase VEGF production. Therefore, CRHR2-induced NO production might affect local VEGF levels, resulting in vessel formation. Hypoxia increases hypoxia inducible factor-1α, which in turn increases VEGF production. In hypertensive CRHR2-deficient mice, vasoconstricted vessels caused tissue hypoxia, which in turn promoted hypoxia inducible factor-1α and VEGF production.
Subsequent studies have investigated the role of the CRH system in tumor angiogenesis. In a mouse xenograft model, CRH-overexpressing epithelial tumor cells increased angiogenesis and tumor growth by stimulating endothelial chemotaxis, without a discernible effect on SMC migration.31 Ucn inhibited the growth of hepatocellular carcinoma by suppressing angiogenesis via the activation of CRHR2.32 In addition, Ucn/CRHR2 was implicated in human renal cell carcinoma, a hypervascularized tumor. In the epithelial cells and microvasculature within the tumor tissues, CRHR2 expression was almost completely lost, indicating that CRHR2/Ucn might act as an endogenous inhibitor of angiogenesis and that disruption of CRHR2/Ucn might promote tumorigenesis.33 In studies of prostate cancer, although Ucn expression did not differ in cancer and normal tissues, CRHR2 expression was detected in the vascular endothelial cells of normal prostate tissue, but not in prostate cancer tissue.34 Thus, loss of CRHR2 expression in the prostate could contribute to cancer progression and increased neovascularization. Furthermore, Ucn II suppressed the growth of Lewis lung carcinoma cell tumors by directly inhibiting cell cycling and suppressing tumor vascularization.35
Peripheral vessel changes, including increased permeability and/or angiogenesis, are a major component of the inflammatory process. The CRH system modulates these events. For instance, CRH and Ucn triggered skin mast cell degranulation and increased vascular permeability, as shown by paw edema assays.36,37 By modulating peripheral vessels, Ucn inhibited paw edema with 6-7 times greater potency than CRH.38 CRHR1 exerted vasoprotective effects during vascular inflammation: blockade of CRHR1 significantly increased the tumor necrosis factor-induced expression of vascular adhesion molecule-1 and E-selectin in human aortic endothelial cells.39 Furthermore, our group concluded that CRHR1 and CRHR2 have opposing effects on intestinal angiogenesis because CRH/CRHR1 promoted endogenous and inflammatory vessel growth, whereas Ucn III/CRHR2 inhibited these responses.7
The CRH family is a potent regulator of cardiovascular function. Intracerebroventricular administration of CRH causes cardiovascular changes similar to stress-induced responses, such as elevation of blood pressure (mean arterial pressure) and heart rate. Systemic administration of CRH or Ucn I might oppose these CNS effects and thus decrease blood pressure by increasing vasodilation in specific vascular beds.13,40 In addition, intracerebroventricular injection of Ucn II and Ucn III increased mean arterial blood pressure and heart rate; the cardiovascular effects of Ucn II were weaker than those induced by Ucn III.41 Furthermore, Ucns were reported to have a protective effect in myocardial infarction and heart failure, possibly through the activation of CRHR2.42 Ucn II and Ucn III protected cardiomyocytes from hypoxia and reduced infarct size by activating the antiapoptotic Erk1/2 pathway.43 Ucn I inhibited reactive oxygen species production and thus protected endothelial cells from free radical damage.44
CRHR2 is expressed in cardiac myocytes and the systemic vasculature. Peripheral vasodilation may activate CRHR2 in vascular endothelial cells and SMCs. CRHR2-null mice exhibited elevated mean arterial pressure and diastolic pressure when compared with wild-type mice, suggesting that changes in cardiac function and blood pressure are critically dependent on CRHR2.45 CRHR2-deficient mice are hypertensive despite having increased blood vessel density; loss of the vasodilatory effect and an increased number of small resistance vessels may contribute to the hypertension.25,45
The mechanism by which the CRH system regulates vasodilation is not yet clear. Vasodilation may be mediated by the release of NO from the endothelium and the consequent activation of the guanylyl cyclase signaling pathway.46 Alternatively, endothelium-independent relaxation may occur through activation of cAMP-dependent protein kinase A, which reduces the calcium sensitivity of contraction.47
This review outlined the previously underappreciated role of the CRH system in the endogenous development of the vasculature and in inflammatory and tumor angiogenesis. Given this role, the CRH system may have a therapeutic application in the treatment of vascular diseases (Fig. 2). Animal and in vitro studies suggest that Ucns have cardioprotective effects, although solid clinical evidence is lacking. Unlike conventional angiogenic factors such as basic fibroblast growth factor and VEGF, the CRH family does not trigger angiogenesis in all settings. Like angiopoietin-1, a potent angiogenic factor in the skin, the CRH family does not stimulate corneal neovascularization, which suggests that the CRH family specifically affects certain vascular beds.48 A recent preclinical study using a synthetic CRH, corticorelin acetate (CrA), showed that CrA significantly delayed tumor growth when administered as a single agent, possibly by inhibiting tumor angiogenesis.49 Moreover, when administered in conjunction with bevacizumab, an anti-VEGF antibody, CrA improved therapeutic outcomes. Because on-going pre-clinical studies point toward the use of the CRH family members as anti-angiogenic agents, additional studies are warranted to establish the mechanistic basis for novel therapeutic approaches to the treatment vascular diseases.
References
1. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981; 213:1394–1397. PMID: 6267699.
2. Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007; 117:33–40. PMID: 17200704.
3. Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988; 94:598–602. PMID: 3257450.
4. Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988; 95:1510–1517. PMID: 2846402.
5. Stengel A, Tache Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood). 2010; 235:1168–1178. PMID: 20881321.
6. Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002; 301:611–617. PMID: 11961064.
7. Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010; 138:2457–2467. PMID: 20206175.
8. Anton PM, Gay J, Mykoniatis A, et al. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci U S A. 2004; 101:8503–8508. PMID: 15159534.
9. Kokkotou E, Torres D, Moss AC, et al. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006; 177:3355–3361. PMID: 16920976.
10. Million M, Tache Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol. 1999; 276:G1027–G1036. PMID: 10198347.
11. Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharideinduced endotoxin shock in mice. Infect Immun. 2002; 70:6068–6074. PMID: 12379683.
12. Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997; 11:457–465. PMID: 9194526.
13. Vaughan J, Donaldson C, Bittencourt J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995; 378:287–292. PMID: 7477349.
14. Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001; 98:7570–7575. PMID: 11416224.
15. Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001; 98:2843–2848. PMID: 11226328.
16. Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999; 115:1–22. PMID: 10375459.
17. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003; 55:21–26. PMID: 12615952.
18. Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropinreleasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995; 373:427–432. PMID: 7830793.
19. Vetter DE, Li C, Zhao L, et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002; 31:363–369. PMID: 12091910.
20. Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999; 415:285–312. PMID: 10553117.
21. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006; 27:260–286. PMID: 16484629.
22. Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010; 57:1–13. PMID: 20234885.
23. Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001; 7:605–611. PMID: 11329063.
24. Smith GW, Aubry JM, Dellu F, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998; 20:1093–1102. PMID: 9655498.
25. Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000; 24:410–414. PMID: 10742108.
26. Nishimura E, Billestrup N, Perrin M, Vale W. Identification and characterization of a pituitary corticotropin-releasing factor binding protein by chemical cross-linking. J Biol Chem. 1987; 262:12893–12896. PMID: 2820956.
27. Chen AM, Perrin MH, Digruccio MR, et al. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A. 2005; 102:2620–2625. PMID: 15701705.
28. Bale TL, Giordano FJ, Hickey RP, et al. Corticotropin-releasing factor receptor 2 is a tonic suppressor of vascularization. Proc Natl Acad Sci U S A. 2002; 99:7734–7739. PMID: 12032352.
29. Bale TL, Giordano FJ, Vale WW. A new role for corticotropin-releasing factor receptor-2: suppression of vascularization. Trends Cardiovasc Med. 2003; 13:68–71. PMID: 12586442.
30. Tsurumi Y, Murohara T, Krasinski K, et al. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997; 3:879–886. PMID: 9256279.
31. Arbiser JL, Karalis K, Viswanathan A, et al. Corticotropin-releasing hormone stimulates angiogenesis and epithelial tumor growth in the skin. J Invest Dermatol. 1999; 113:838–842. PMID: 10571742.
32. Wang J, Xu Y, Zhu H, Zhang R, Zhang G, Li S. Urocortin's inhibition of tumor growth and angiogenesis in hepatocellular carcinoma via corticotrophin-releasing factor receptor 2. Cancer Invest. 2008; 26:359–368. PMID: 18443956.
33. Tezval H, Jurk S, Atschekzei F, et al. Urocortin and corticotropinreleasing factor receptor 2 in human renal cell carcinoma: disruption of an endogenous inhibitor of angiogenesis and proliferation. World J Urol. 2009; 27:825–830. PMID: 19437022.
34. Tezval H, Jurk S, Atschekzei F, Serth J, Kuczyk MA, Merseburger AS. The involvement of altered corticotropin releasing factor receptor 2 expression in prostate cancer due to alteration of antiangiogenic signaling pathways. Prostate. 2009; 69:443–448. PMID: 19058138.
35. Hao Z, Huang Y, Cleman J, et al. Urocortin2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci U S A. 2008; 105:3939–3944. PMID: 18308934.
36. Singh LK, Boucher W, Pang X, et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999; 288:1349–1356. PMID: 10027877.
37. Theoharides TC, Singh LK, Boucher W, et al. Corticotropinreleasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998; 139:403–413. PMID: 9421440.
38. Turnbull AV, Vale W, Rivier C. Urocortin, a corticotropin-releasing factor-related mammalian peptide, inhibits edema due to thermal injury in rats. Eur J Pharmacol. 1996; 303:213–216. PMID: 8813571.
39. Inada Y, Ikeda K, Tojo K, Sakamoto M, Takada Y, Tajima N. Possible involvement of corticotropin-releasing factor receptor signaling on vascular inflammation. Peptides. 2009; 30:365–372. PMID: 19026699.
40. Overton JM, Fisher LA. Differentiated hemodynamic responses to central versus peripheral administration of corticotropinreleasing factor in conscious rats. J Auton Nerv Syst. 1991; 35:43–51. PMID: 1940026.
41. Jin R, Li MZ, Bing YH, et al. Intracerebroventricular injection of stresscopin-related peptide enhances cardiovascular function in conscious rats. Regul Pept. 2013; 186:7–11. PMID: 23850799.
42. Brar BK, Jonassen AK, Egorina EM, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004; 145:24–35. PMID: 12970163.
43. Brar BK, Jonassen AK, Stephanou A, et al. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000; 275:8508–8514. PMID: 10722688.
44. Barry SP, Lawrence KM, McCormick J, et al. New targets of urocortin-mediated cardioprotection. J Mol Endocrinol. 2010; 45:69–85. PMID: 20501665.
45. Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000; 24:403–409. PMID: 10742107.
46. Jain V, Vedernikov YP, Saade GR, Chwalisz K, Garfield RE. Endothelium-dependent and -independent mechanisms of vasorelaxation by corticotropin-releasing factor in pregnant rat uterine artery. J Pharmacol Exp Ther. 1999; 288:407–413. PMID: 9918539.
47. Lubomirov L, Gagov H, Petkova-Kirova P, Duridanova D, Kalentchuk VU, Schubert R. Urocortin relaxes rat tail arteries by a PKA-mediated reduction of the sensitivity of the contractile apparatus for calcium. Br J Pharmacol. 2001; 134:1564–1570. PMID: 11724764.
48. Suri C, McClain J, Thurston G, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998; 282:468–471. PMID: 9774272.
49. Gamez I, Ryan RP, Reid LD, Routt SM, Hollister BA. Corticorelin acetate, a synthetic corticotropin-releasing factor with preclinical antitumor activity, alone and with bevacizumab, against human solid tumor models. Cancer Chemother Pharmacol. 2011; 67:1415–1422. PMID: 20809121.
50. Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-proteincoupled receptor CRFR1. J Biol Chem. 2008; 283:32900–32912. PMID: 18801728.
51. Grace CR, Perrin MH, Cantle JP, Vale WW, Rivier JE, Riek R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J Am Chem Soc. 2007; 129:16102–16114. PMID: 18052377.
Fig. 1
Overview of the corticotropin-releasing hormone (CRH) system. The CRH system is composed of natural ligands (CRH and urocortin [Ucn] I-III), receptors (CRHR1 and 2), and binding proteins (CRH-BP and sCRHR2α). CRH and Ucn I preferentially bind to CRHR1, whereas Ucn II and Ucn III exclusively bind to CRHR2. CRH-BP and sCRHR2α bind to CRH and Ucn I to modulate the biological activities of the ligands. Human CRH (protein data bank [PDB] entry 3EHU);50 Ucn I (PDB entry 2RMF);51 Ucn II (PDB entry 2RMG);51 Ucn III (PDB entry 2RMH).51
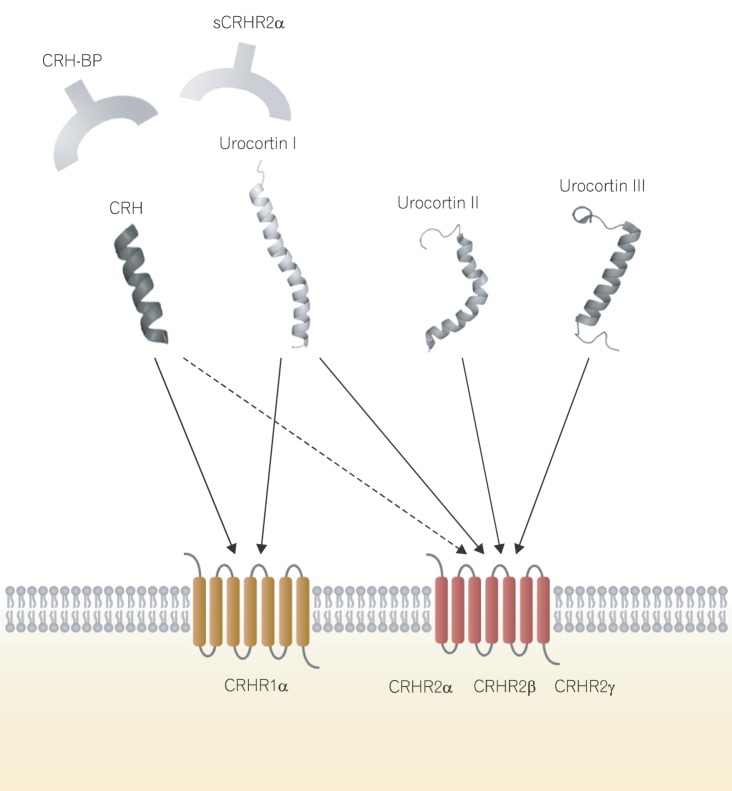
Fig. 2
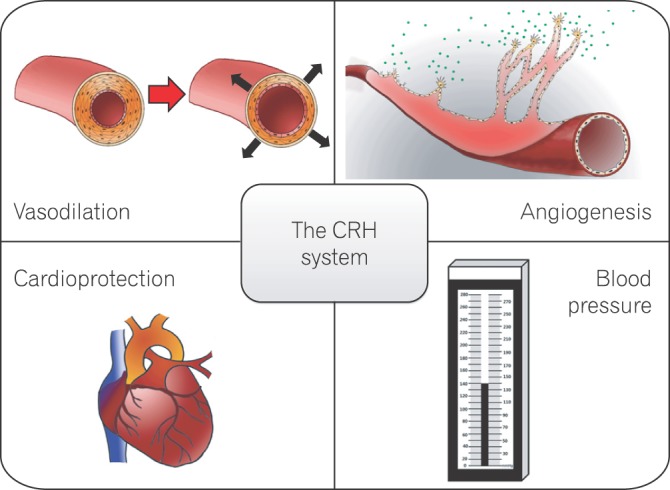
Functional diversity of the corticotropin-releasing hormone (CRH) system in relation to angiogenesis. The CRH system has been implicated in various biological functions pertinent to the cardiovascular system, including angiogenesis, vasodilation, alteration of blood pressure, and cardioprotection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download