Abstract
Background/Aims
Colorectal cancer (CRC) develops from colonic adenomas. Type 2 diabetes mellitus (DM) is associated with a higher risk of CRC and metformin decreases CRC risk. However, it is not certain if metformin affects the development of colorectal polyps and adenomas. This study aimed to elucidate if metforminaffects the incidence of colonic polyps and adenomas in patients with type 2 DM.
Methods
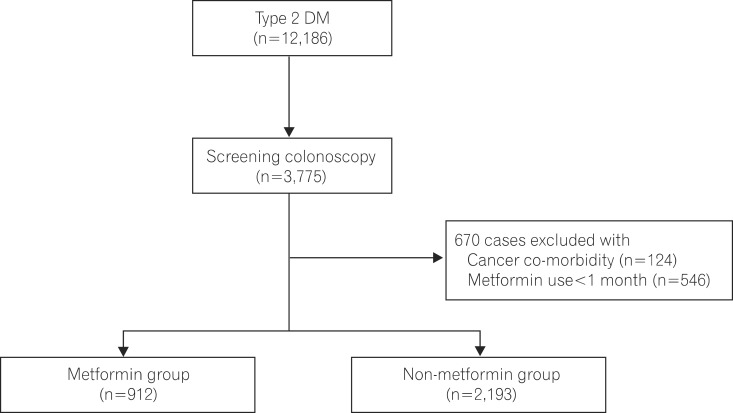
Of 12,186 patients with type 2 DM, 3,775 underwent colonoscopy between May 2001 and March 2013. This study enrolled 3,105 of these patients, and divided them in two groups: 912 patients with metformin use and 2,193 patients without metformin use. Patient clinical characteristics, polyp and adenoma detection rate in the two groups were analyzed retrospectively.
Results
The Colorectal polyp detection rate was lower in the metformin group than in the non-meformin group (39.4% vs. 62.4%, P<0.01). Colorectal adenoma detection rate was significantly lower in the metformin group than in the non-metformin group (15.2% vs. 20.5%, P<0.01). Fewer advanced adenomas were detected in the metformin group than in the non-metformin group (12.2% vs. 22%, P<0.01). Multivariate analysis identified age, sex, Body mass index and metformin use as factors associated with polyp incidence, whereas only metforminwas independently associated with decreased adenoma incidence (Odd ratio=0.738, 95% CI=0.554-0.983, P=0.03).
Go to : 
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths worldwide,1 and its prevalence and mortality rates continue to increase in Korea.2 CRC mostly develops from colonic adenomas.3 To reduce the incidence of CRC, the focus has shifted from removal of early-detected adenomas to prevention of the formation of adenomas causing CRC; recent studies have investigated the effects of dietary fiber, calcium, nonsteroidal anti-inflammatory drugs, aspirin, statins, and other medications in the prevention of CRC.4,5 Since CRC is a cancer associated with lifestyle-related diseases such as diabetes mellitus (DM) or obesity, the therapeutic agents used for these diseases have been investigated for the prevention of CRC.6,7 Type 2 DM is a known risk factor for CRC worldwide;8 hyperinsulinemia caused by insulin resistance influences the incidence of CRC.9,10,11 Metformin is a biguanide derivative that inhibits gluconeogenesis and glycogen decomposition, and increases glucose absorption in muscle tissues. Unlike other oral hypoglycemic agents, metformin does not cause hypoglycemia; therefore, it is appropriate for the initial treatment of diabetes.12 Metformin also activates liver kinase B1-dependent AMP-activated protein kinase (AMPK) in the liver, and activated AMPK inhibits the proliferation and growth of cells by inhibiting mammalian target of rapamycin.13 A recent study suggests that AMPK activated by low-dose metformin inhibits the formation of aberrant crypt foci (ACF), a surrogate marker of CRC.14,15 Moreover, it has also been recently reported that the risk of cancers, including CRC, is lower in type 2 DM patients being treated with metformin than those not treated with metformin.9,16,17,18,19,20 Some animal studies have revealed that metformin prevents the proliferation of colonic epithelial cells.21,22 However, it remains to be proven whether the intake of metformin inhibits the occurrence of adenomas, which are the precursor lesions of CRC. In this study, we aimed to investigate if administrating metformin prevented the formation of colonic polyps, particularly adenomas, in patients with type 2 DM.
Go to : 
This study retrospectively reviewed the records of 3,775 patients aged ≥40 years with no family history of CRC and who underwent colonoscopy for the first time from a total cohort of 12,186 patients diagnosed with type 2 DM from May 2001 to March 2013 in Soonchunhyang University Bucheon Hospital. We excluded 124 patients with malignant neoplasm, IBD, or cancers other than CRC, and 546 patients who were administered metformin for <1 month prior to colonoscopy. Adenoma was confirmed based on the pathological findings of endoscopic biopsy. This study enrolled 3,105 patients (Fig. 1), and was performed after approval from the institutional review board of Soonchunhyang University Bucheon Hospital (SCHBC_IRB_2013-64).
We recorded the age, sex, BMI, any associated illnesses, duration of DM, smoking status, use of aspirin and statins, duration of metformin use, and blood test results (triglyceride [TG], total cholesterol, HDL cholesterol, LDL cholesterol, and glycated hemoglobin [HbA1c] levels) during colonoscopy. Since the use of low-dose metformin for >1 month could inhibit the formation of ACF, patients were divided into those with a history of metformin use for >1 month prior to colonoscopy (the metformin group) and those with no history of metformin use (non-metformin group). Polyps detected during colonoscopy were divided into hyperplastic polyps, adenomas, inflammatory polyps, and adenocarcinomas according to histologic findings. Adenomas were classified into tubular, tubulovillous, and villous adenomas according to histologic types, and low grade, moderate grade, and high-grade dysplasias according to differentiation. Adenomas >1 cm, tubulovillous or villous adenomas, high-grade dysplasia, or more than three small-sized adenomas were defined as advanced adenomas.23,24
All statistical analyses were performed using the statistical software package SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). All data were presented as mean±SD, numerical values, and percentages. The analysis of continuous variables was conducted using Student t-test, whereas the analysis of categorical variables was performed using the chi-square test. Univariate analysis was performed to compare the polyp detection rate (PDR) and adenoma detection rate (ADR) between the metformin and non-metformin groups. This study investigated if metformin affects the PDRs and ADRs using multivariate logistic regression analysis to modify variables with statistical difference such as age, sex, BMI, and TG levels, and variables that might confound analysis results including duration of DM, use of aspirin and statins, and smoking history. P-values>0.05 were considered statistically significant.
Go to : 
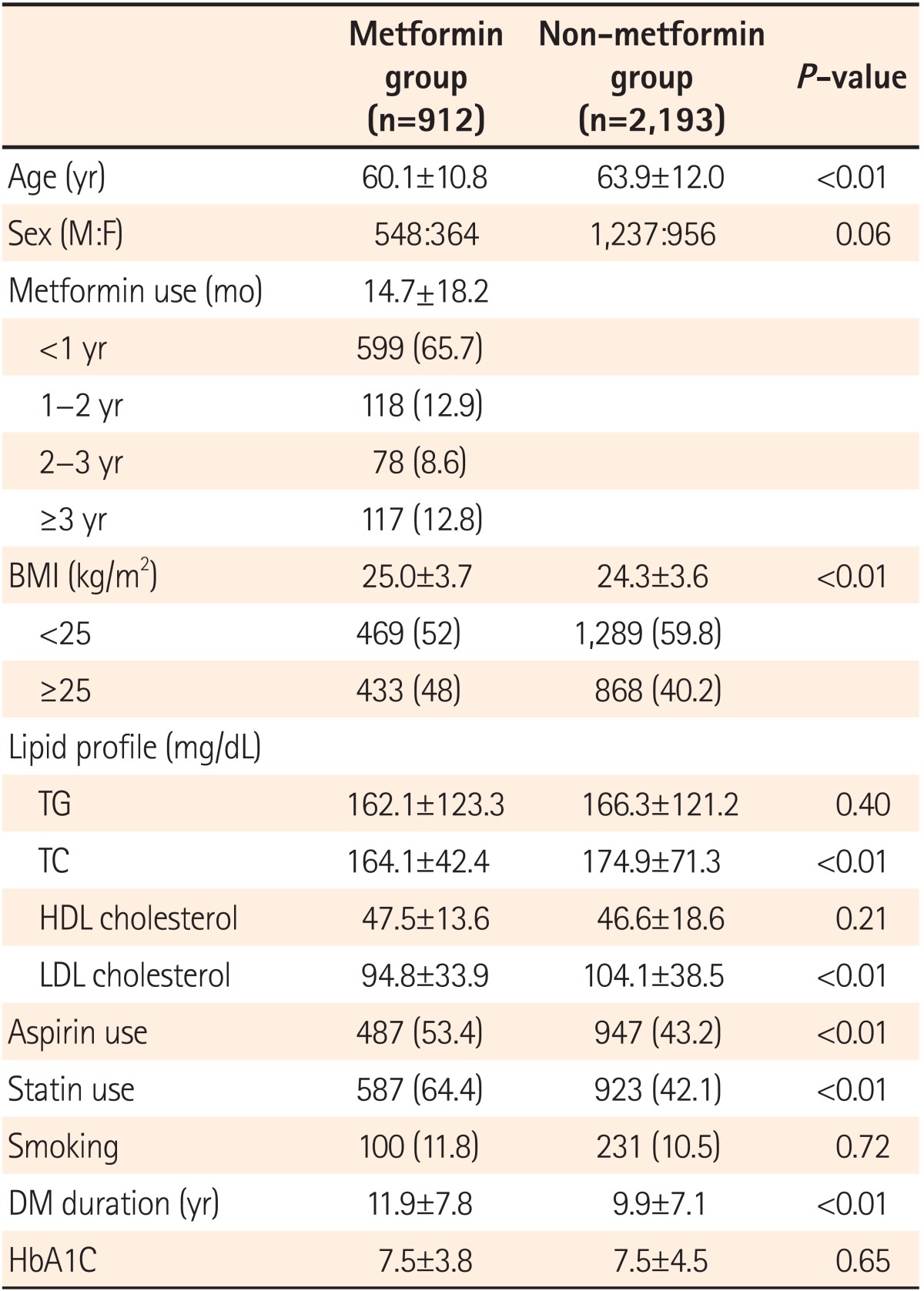
A total of 3,105 patients were enrolled in the study, with 912 patients in the metformin group and 2,193 patients in the non-metformin group. Mean duration of metformin medication use was 14.7±18.2 months. Patients with metformin use for <1 year accounted for 65.7%, and 12.8% of all subjects used metformin for >3 years. The mean age of patients was significantly lower in the metformin group than in the non-metformin group (60.1±10.8 vs. 63.9±12.0 years, P<0.01). BMI was recorded to determine obesity. Mean BMI was significantly higher in metformin group than in the non-metformin group; it was in the metformin group (25.0±3.7 vs. 24.3±3.6 kg/m2, P<0.01). The metformin group had a larger proportion of patients with BMI >25 kg/m2 than the non-metformin group (48% vs. 40.2%, P<0.01). The proportion of smokers were 11% and 10.5% in the metformin and non-metformin groups, respectively, with no statistical difference between the two groups (P=0.72). The time since diagnosis of DM was longer in the metformin group than in the non-metformin group (11.9±7.8 vs. 9.9±7.1 years, P<0.01). Both total cholesterol and LDL cholesterol levels were higher in the non-metformin group than in the metformin group. No statistical difference was found in HbA1c between the two groups (7.5±3.8 vs. 7.5±4.5 mmol/mol, P=0.65). The proportion of patients using aspirin and statins were significantly higher in the metformin group than in the non-metformin group (aspirin: 53.4%, statins: 64.4%, P<0.01; Table 1).
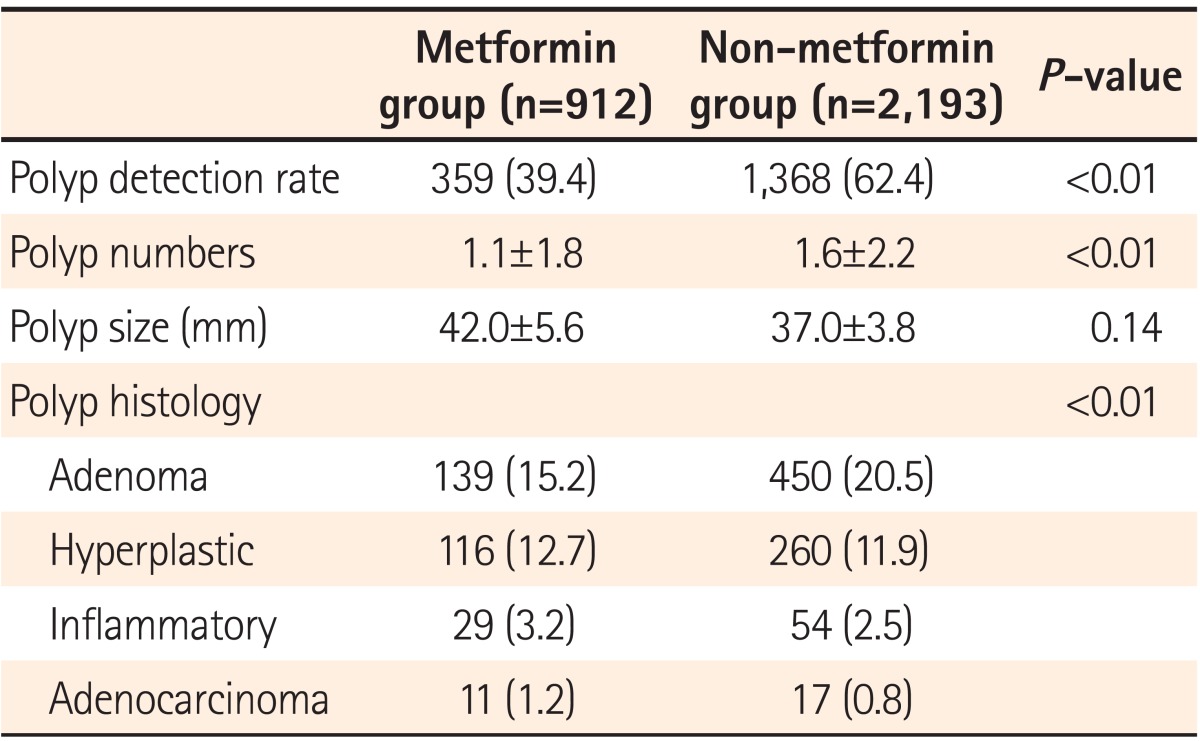
Polyp detection rates (PDRs) were 39.4% and 62.4% in metformin and non-metformin groups, respectively, and were significantly higher in the non-metformin group (P<0.01). The mean number of polyps detected was significantly higher in the non-metformin group than in the metformin group (1.6±2.2 vs. 1.1±1.8, P<0.01). ADRs were 15.2% and 20.5% in the metformin and non-metformin groups, respectively, showing a statistically significantly higher detection rate in the non-metformin group (P<0.01). On the other hand, polyp size did not significantly differ between the groups (42.0±5.6 vs. 37.0±3.8 mm, P=0.14; Table 2).
The detection rates of polyps and adenomas were compared in the metformin group according to the duration of metformin use. The PDR was 36.6% in patients taking metformin for <1 year, 47.5% in patients taking metformin for 1-2 years, 42.3% in patients taking metformin for 2-3 years, and 43.6% in patients taking metformin for >3 years, with no statistically significant difference among groups (P=0.07). The ADR was 15.9% in patients taking metformin for <1 year, 13.6% in patients taking metformin for 1-2 years, 14.1% in patients taking metformin for 2-3 years, and 14.5% in patients taking metformin for >3 years, with no statistically significant difference among groups (P=0.59; Fig. 2).
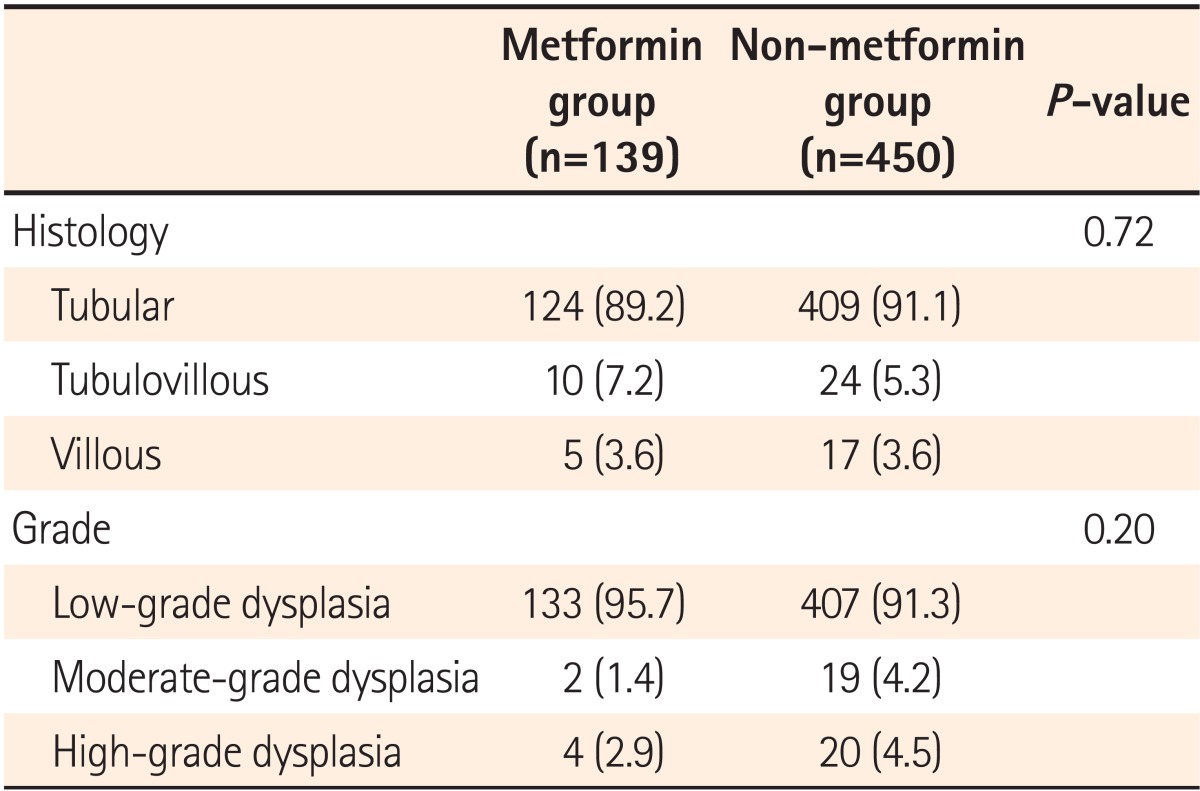
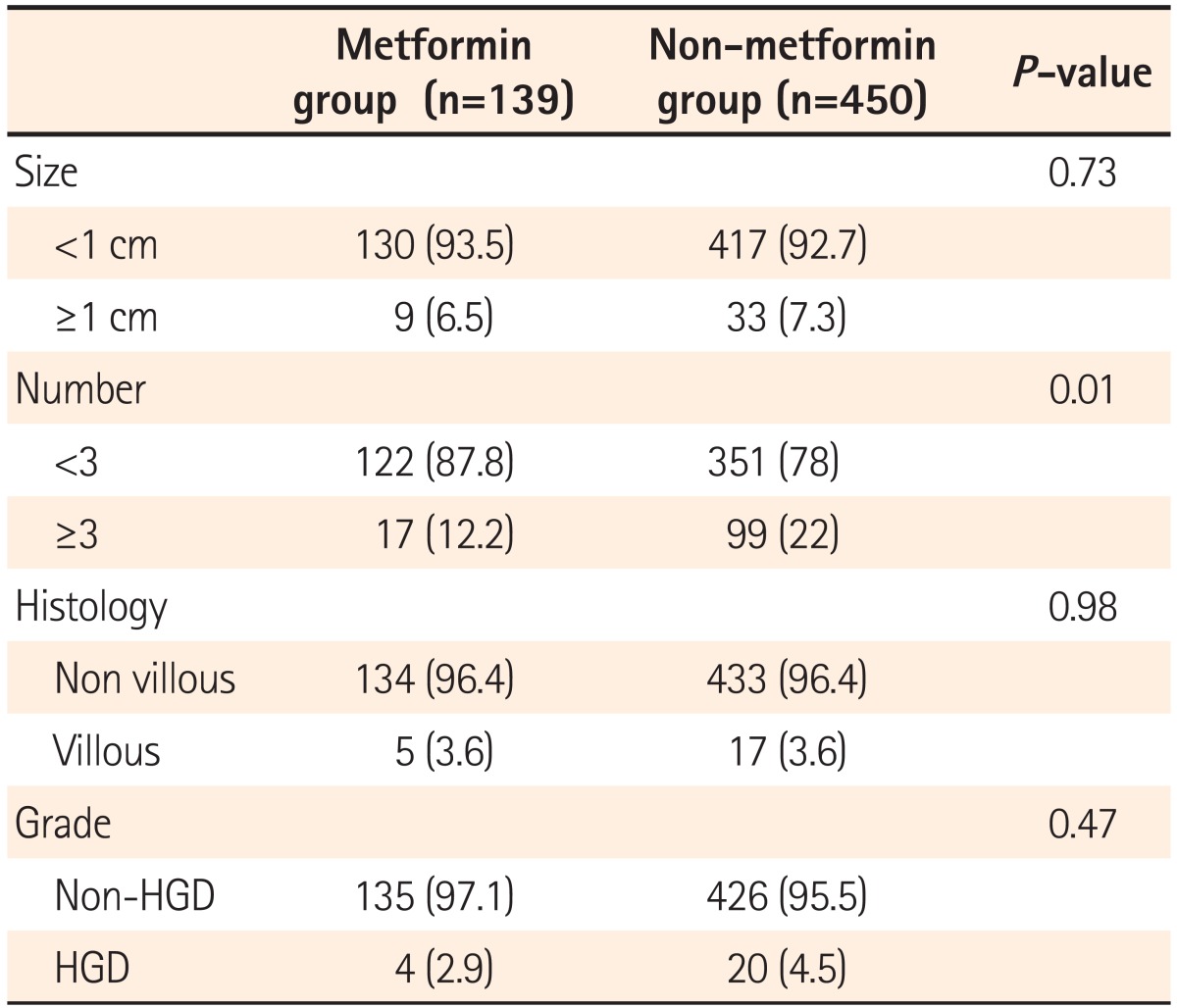
Histologic classification and grade were compared between adenomas detected in the metformin and non-metformin groups. No difference was found in the ratios of tubular, tubulovillous, and villous adenomas between the two groups (P=0.72), or in the proportion of low grade, moderate grade, and high-grade dysplasias (P=0.20; Table 3).
Among the study subjects, 6.5% and 7.3% had adenomas >1 cm in the metformin and non-metformin groups, respectively, with no statistically significant difference between the two groups (P=0.73). No statistical difference was found in the detection rate of villous adenomas between the two groups (P=0.98). The detection rate of well-differentiated adenomas was lower in metformin group than in the non-metformin group, with no significant difference between the two groups (2.9% vs. 4.5%, P=0.47). The detection rate of more than three adenomas with a higher recurrence rate was markedly lower in metformin group than in the non-metformin group (12.2% vs. 22%, P<0.01; Table 4).
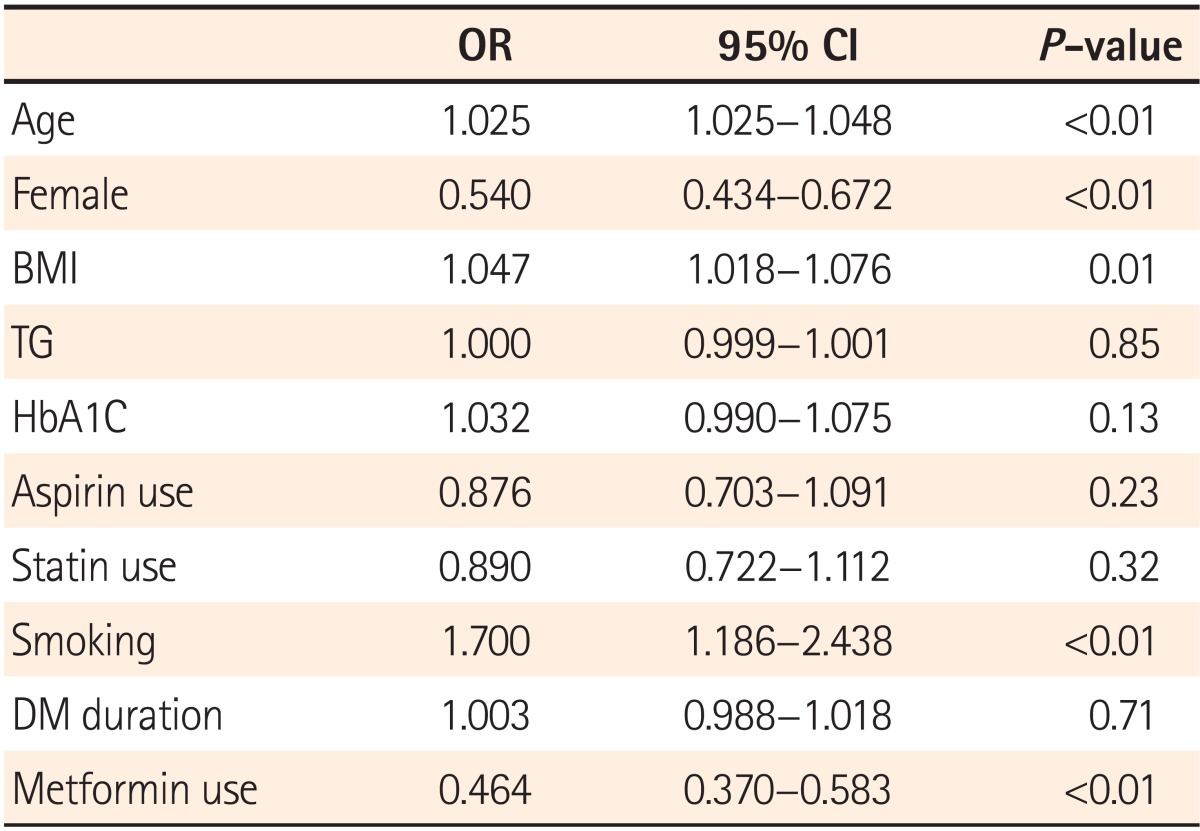
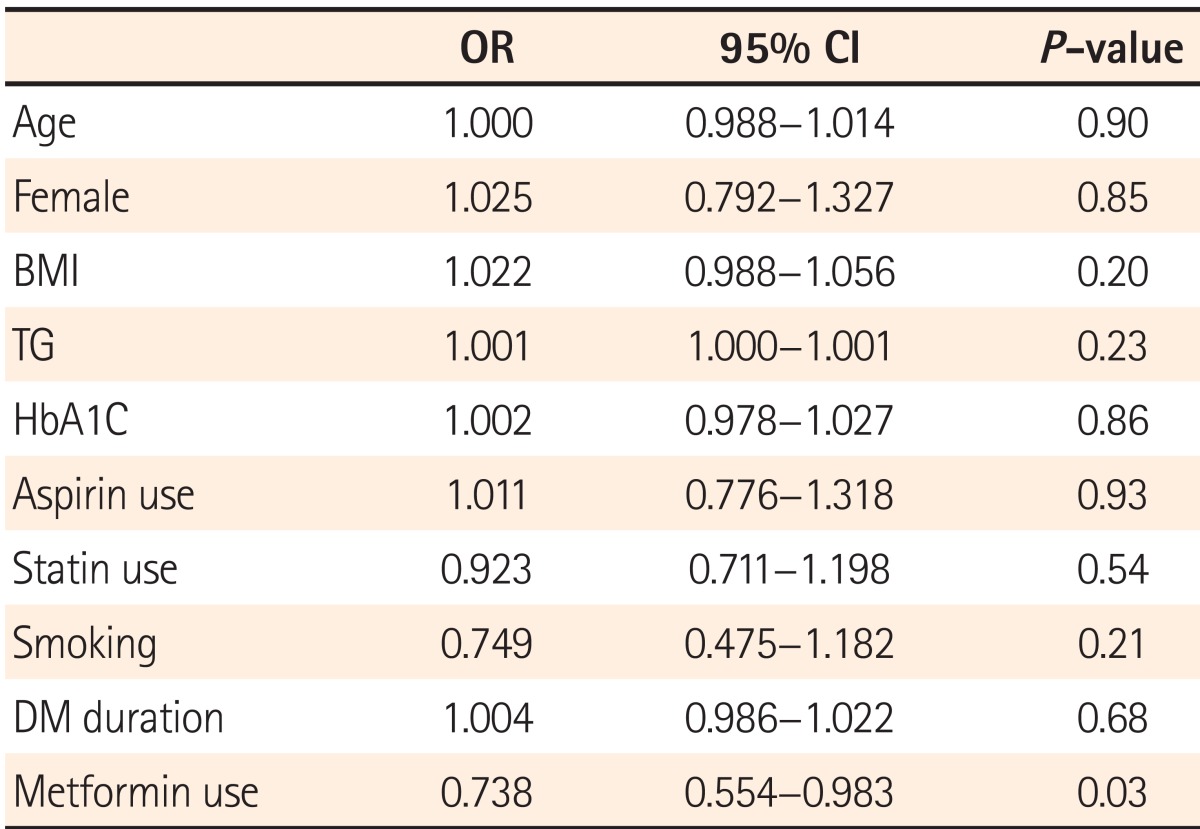
Regression analysis was performed to identify whether the use of metformin is the factor that affects the detection rate of polyps and adenomas by modifying variables including age, sex, BMI, TG, HbA1c, duration of DM, smoking status, and use of aspirin and statins. According to the results, age, sex, BMI, and smoking status were identified as factors influencing the PDR, in addition to metformin use (Table 5). Regression analysis conducted by adjusting the above variables to identify factors affecting the ADR showed that metformin use alone reduced the detection rate of adenomas (OR=0.738, 95% CI=0.554-0.983, P=0.03; Table 6).
Go to : 
A number of studies have shown that DM is associated with an increased incidence of CRC, breast cancer, endometrial cancer, renal cancer, liver cancer, and other cancers;25 meta-analyses have proven that the relative risk of CRC is higher in patients with DM.26 Insulin-like growth factor-1 (IGF-1) level increases due to hyperinsulinemia resulting from insulin resistance in DM patients, and elevated IGF-1 levels affect the incidence of colonic adenomas and CRC inducing proliferation and dysplasia of normal and carcinoma cells.27 The incidence and mortality rates of CRC are showing a continuously increasing trend worldwide.2 Along with the removal of early-detected CRC or colonic adenomas, the use of preventive medicines is anticipated to be effective in preventing CRC. The most common cause of CRC is development from colonic adenomas after a series of genetic variations. Although the association of DM with colonic adenomas has been verified,28,29 and metformin has been proven to lower the risk of CRC in patients with type 2 DM,30 results of different studies that have investigated whether metformin decreases the incidence of colonic polyps or adenomas are inconsistent. A Korean study showed that metformin reduced the incidence of colonic adenomas in patients with a history of CRC.31 For these reasons, this study compared the detection rate of colonic polyps and adenomas in 3,105 patients with type 2 DM aged ≥40 years with no history of CRC and who were undergoing colonoscopy for the first time by dividing them into two groups: a metformin and non-metformin group. The detection rates of both polyps and adenomas were found to be significantly lower in the metformin group than in the non-metformin group, implying that metformin prevents the incidence of colonic polyps and the occurrence of colonic adenomas, which are precursor lesions of CRC. Although no difference was found in the histologic classification and grade of colonic adenomas between the two groups, the incidence of three colonic adenomas was significantly lower in the metformin group than in the non-metformin group. According to the multivariate analysis performed to identify factors that affect the detection rate of colonic adenomas, age, sex, BMI, TG, HbA1c, smoking status, and DM duration were not found to be associated with the incidence of colonic adenomas. Unlike previous studies that suggested that the use of aspirin and statins was related with the occurrence of colonic adenomas,32,33 the risk of colonic adenomas was not reduced in patients treated with aspirin or statins in this study. In contrast, metformin use decreased the risk of colonic adenoma incidence by 37%. Therefore, metformin may be effective in preventing the development of colonic adenomas in patients with type 2 DM. This study has a few limitations. It was a single-center retrospective study. Since the study mainly relied on medical records without evaluating the clinical characteristics of patients or the use of aspirin or statins, the duration of medication use was indeterminable. Therefore, the outcome of this study cannot be compared with that of previous studies. Furthermore, the skill-level of physicians who performed colonoscopy for polyp detection was not standardized, and colon cleanliness and colonoscopy withdrawal time were not evaluated as variables that may have affected the detection rate. Unlike previous studies, this study identified the effectiveness of metformin in lowering the occurrence of colonic adenomas in patients with type 2 DM by comparing the detection rates of polyps and adenomas in a large number of subjects with type 2 DM stratified according to metformin use. Large-scale, prospective, randomized studies with various controlled confounding variables will be needed to overcome the study limitations and clearly prove the efficacy of metformin in preventing colonic adenomas.
Go to : 
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29. PMID: 22237781.

2. Anderson WF, Umar A, Brawley OW. Colorectal carcinoma in black and white race. Cancer Metastasis Rev. 2003; 22:67–82. PMID: 12716038.
3. Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974; 67:451–457. PMID: 4853754.
4. Das D, Arber N, Jankowski JA. Chemoprevention of colorectal cancer. Digestion. 2007; 76:51–67. PMID: 17947819.

5. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001; 1:11–21. PMID: 11900248.

6. Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2005; 14:133–137. PMID: 15668486.

7. Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005; 28:1805–1807. PMID: 15983343.

8. Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003; 157:1092–1100. PMID: 12796045.

9. Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009; 52:1766–1777. PMID: 19572116.

10. Seow A, Yuan JM, Koh WP, Lee HP, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst. 2006; 98:135–138. PMID: 16418516.

11. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011; 128:635–643. PMID: 20473855.

12. Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008; 31:2086–2091. PMID: 18782901.

13. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005; 17:596–603. PMID: 16226444.

14. Gupta AK, Pretlow TP, Schoen RE. Aberrant crypt foci: what we know and what we need to know. Clin Gastroenterol Hepatol. 2007; 5:526–533. PMID: 17433788.

15. Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila). 2010; 3:1077–1083. PMID: 20810669.

16. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009; 32:1620–1625. PMID: 19564453.
17. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: response to Farooki and Schneider. Diabetes Care. 2006; 29:1990–1991. PMID: 16873829.

18. Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010; 53:1631–1637. PMID: 20407744.

19. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005; 330:1304–1305. PMID: 15849206.

20. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010; 33:322–326. PMID: 19918015.
21. Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008; 99:2136–2141. PMID: 18803638.

22. Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010; 49:662–671. PMID: 20564343.

23. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003; 124:544–560. PMID: 12557158.

24. Bond JH. Practice Parameters Committee of the American College of Gastroenterology. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Am J Gastroenterol. 2000; 95:3053–3063. PMID: 11095318.

25. Grote VA, Becker S, Kaaks R. Diabetes mellitus type 2 - an independent risk factor for cancer? Exp Clin Endocrinol Diabetes. 2010; 118:4–8. PMID: 20127570.

26. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:1679–1687. PMID: 16288121.

27. Eddi R, Karki A, Shah A, DeBari VA, DePasquale JR. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer. 2012; 43:87–92. PMID: 21894459.

28. Yoshida I, Suzuki A, Vallee M, et al. Serum insulin levels and the prevalence of adenomatous and hyperplastic polyps in the proximal colon. Clin Gastroenterol Hepatol. 2006; 4:1225–1231. PMID: 16979948.

29. Kono S, Honjo S, Todoroki I, et al. Glucose intolerance and adenomas of the sigmoid colon in Japanese men (Japan). Cancer Causes Control. 1998; 9:441–446. PMID: 9794177.
30. Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011; 34:2323–2328. PMID: 21949223.

31. Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012; 44:1042–1047. PMID: 22789400.

32. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010; 376:1741–1750. PMID: 20970847.

33. Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010; 59:1572–1585. PMID: 20660702.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download