Abstract
Background/Aims
Methods
Results
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS: T.H. and C.S. were involved in conception and design of the study. T.H. was also involved in acquisition of data. R.Z. was involved in analysis of data. All authors were involved in interpretation of data, drafting and critical revision of the manuscript and have approved the final manuscript for submission. All authors also had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support: This study was funded by Janssen Research & Development, LLC and Janssen Pharmaceutical K.K., Japan.
Conflict of interest: Yuya Imai, Yoko Murata, Nobuko Matsushima, and Richuan Zheng are employees of Janssen Pharmaceutical K.K., Japan. Dr. Christopher Gasink is an employee of Janssen Research & Development, LLC, United States. Dr. Toshifumi Hibi is a consultant for Janssen Pharmaceutical K.K., Japan.
References
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
Supplementary Fig. 2
Supplementary Fig. 3
Supplementary Fig. 4
Supplementary Fig. 5
Supplementary Fig. 6
Supplementary Fig. 7
Fig. 1
Study design of phase 3 CD program for ustekinumab. Patients randomized to placebo and patients who were nonresponders to ustekinumab were eligible for nonrandomized maintenance dose after completion of induction study. aWeight range based ustekinumab doses approximating at 6 mg/kg; bIf there was no clinical response achieved at week 8, the study treatment was discontinued. TNF, tumor necrosis factor; IV, intravenous; q8w, every 8 weeks; q12w, every 12 weeks. Adapted from Feagan BG, et al. N Engl J Med 2016;375:1946-1960.19
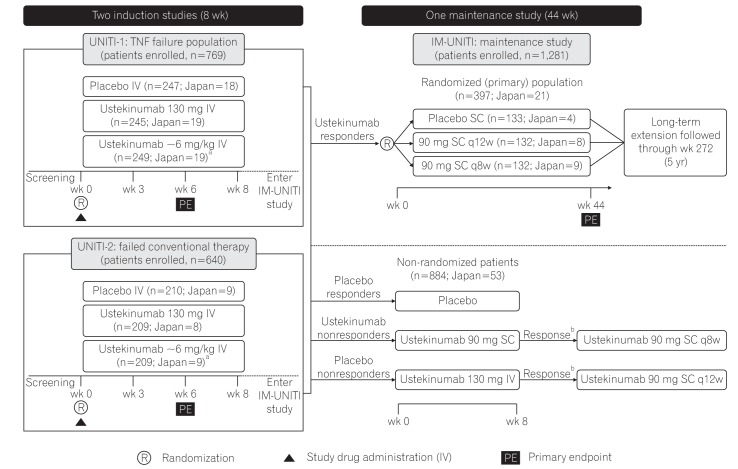
Fig. 2
Primary endpoint in the overall study population and Japanese subpopulation. The primary efficacy outcome in induction and maintenance studies observed in Japanese subpopulation were similar to the global study population. aP<0.001 vs. placebo; bP<0.01 vs. placebo; cP=0.04. q8w, every 8 weeks; q12w, every 12 weeks.
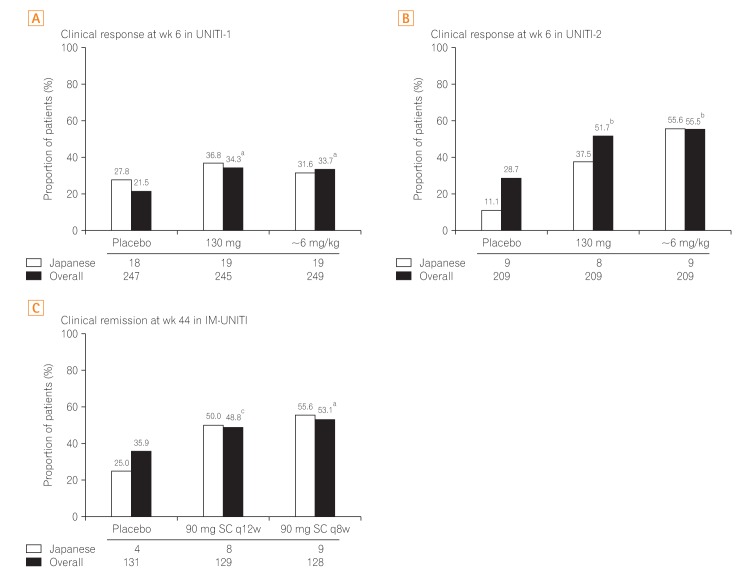
Fig. 3
Median change in CDAI scores over time. In the induction studies and maintenance study, the median change in CDAI scores consistently improved with ustekinumab compared with that with placebo in the Japanese subpopulation and overall study population. All P<0.05 vs. placebo in the overall population of the UNITI-1, UNITI-2, and IM-UNITI studies. q12w, every 12 weeks; q8w, every 8 weeks. Panel B, D, and F are adapted from Feagan BG, et al. N Engl J Med 2016;375:1946-1960, with permission Massachusetts Medical Society.19
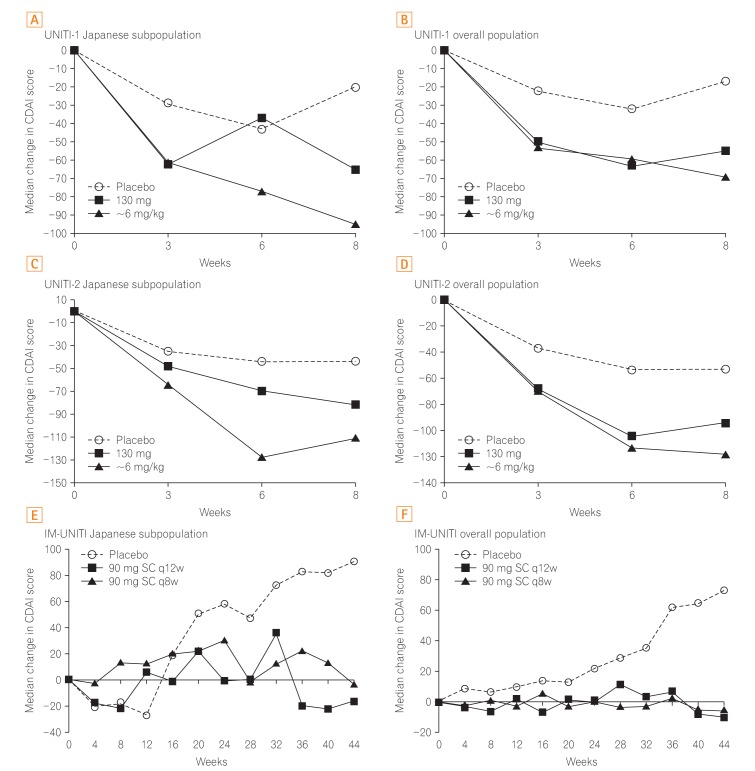
Table 1
Patient Demographics and Clinical Characteristics of the Overall Study Population and Japanese Subpopulation: Induction Studies and Maintenance Study
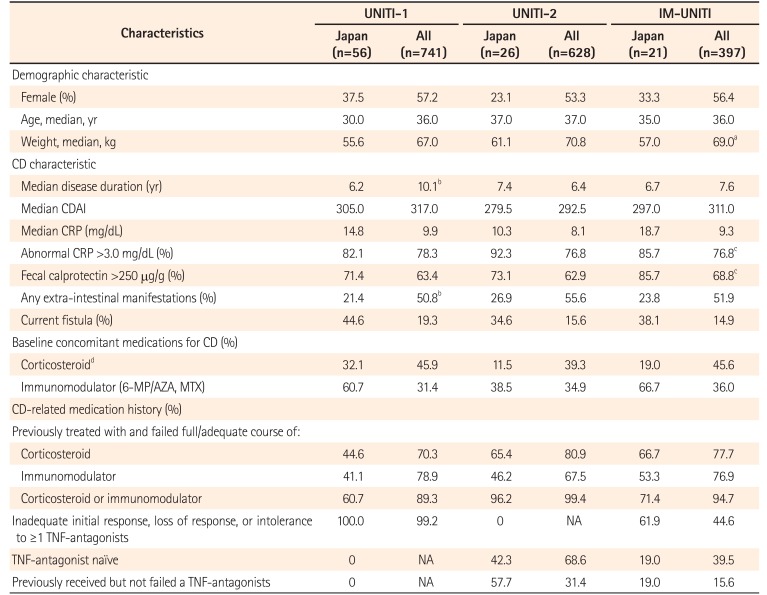
Table 2
Major Secondary Endpoints of Induction Studies in the Overall Population and Japanese Subpopulation
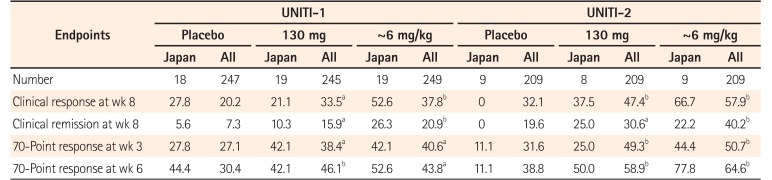
Table 3
Summary of Safety Results through Week 8 (Combined UNITI-1 and-2 Study)
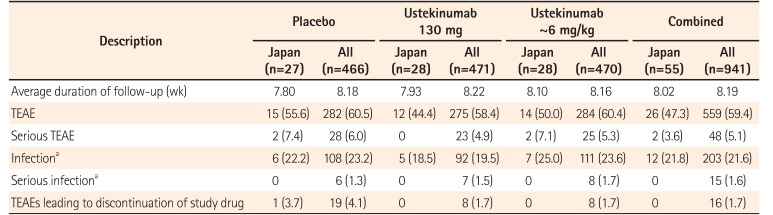
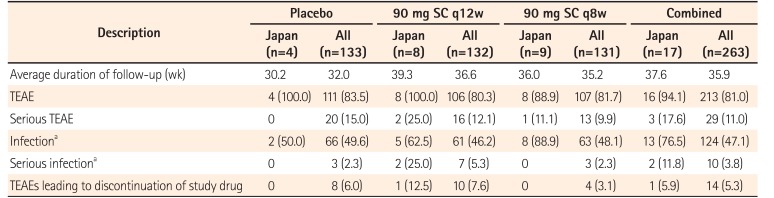
Table 4
Summary of Safety Results through Week 44 of the IM-UNITI Study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download