Abstract
Background/Aims
Methods
Results
Conclusions
Notes
References
Fig. 1
Examples of the spectrum of lesions found in the colon. (A) Endoscopic finding of hyperplastic polyp (HP): a 6-mm transverse colon polyp with a smooth and pale appearance. (B) Endoscopic finding of sessile serrated adenoma (SSA): a 12-mm ascending colon polyp with a flat or sessile appearance and indistinct borders. It also has a characteristic rim of debris. (C) Endoscopic finding of traditional serrated adenoma (TSA): a 15-mm descending colon polyp showing a granulonodular and lobular appearance. (D) Histopathologic finding of HP: serrated crypts are confined to the upper crypt. (E) Histopathologic finding of SSA: serrated surfaces are enlarged and epithelial serration extends to the crypt bases. (F) Histopathologic finding of TSA: serrated crypt with pseudostratified, elongated nuclei and abundant eosinophilic cytoplasm is characteristic (D–F, H&E stain).
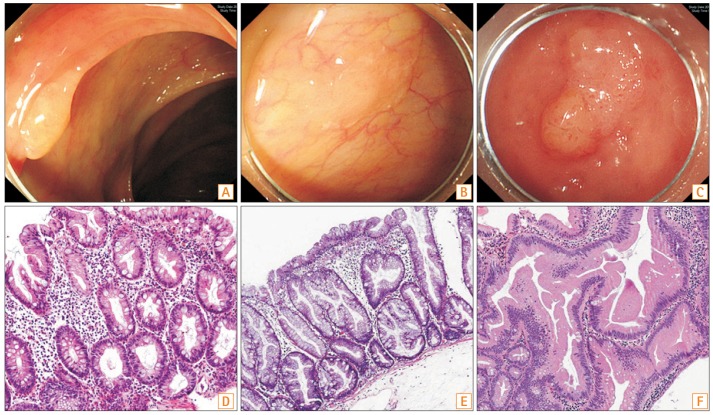
Fig. 2
Distribution of serrated polyps (SPs) according to location. SPs ≥10 mm were found more frequently in the proximal colon, with the majority usually found in the distal colon. Similar to the distribution of SPs ≥10 mm, most serrated adenomas (SAs) were distributed throughout the proximal colon.

Table 1
Demographic and Clinical Characteristics of Patients with SPS
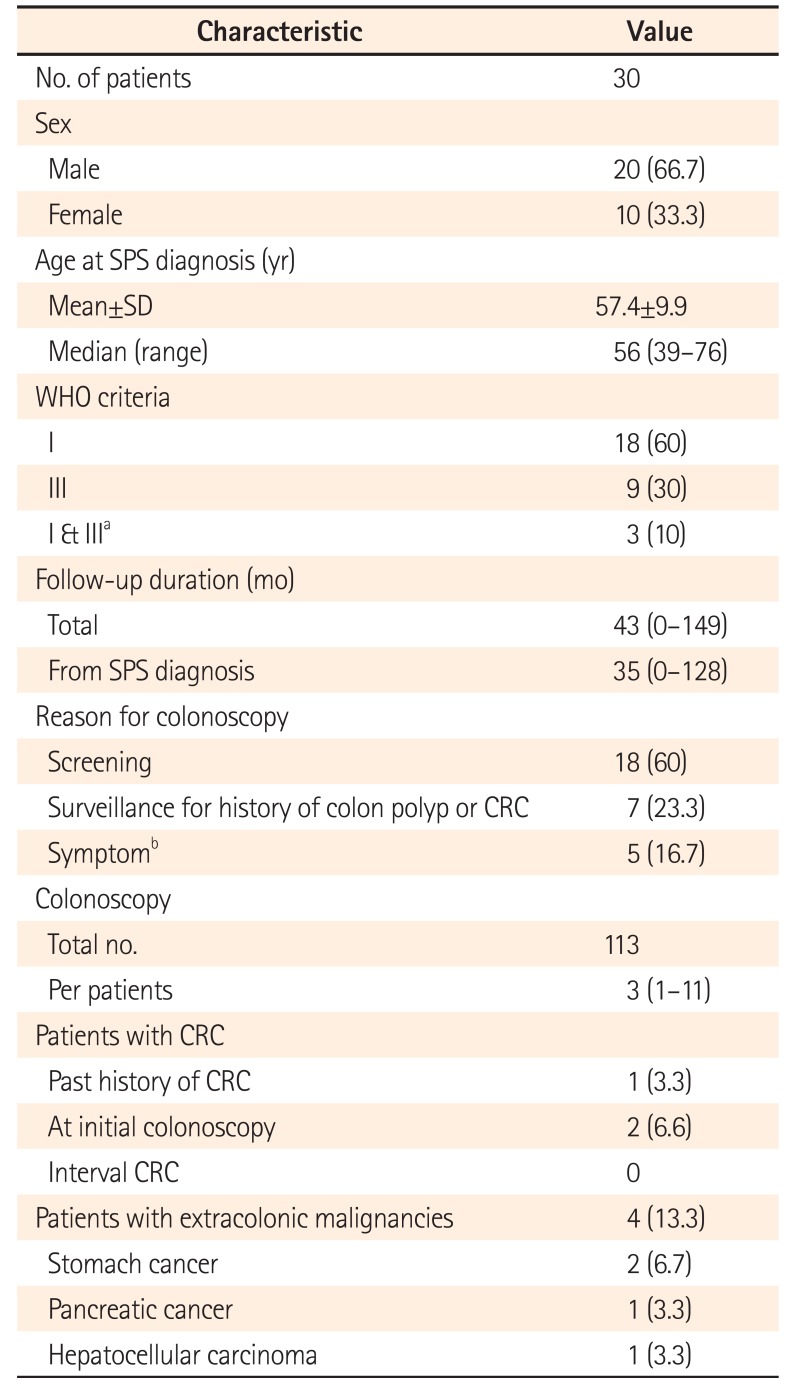
Values are presented as number (%) or median (range).
aThree patients initially diagnosed with SPS by WHO criterion I then met criterion III during surveillance.
bSymptoms included bowel habit changes, abdominal pain, bloody stool, and/or anemia.
SPS, serrated polyposis syndrome; WHO, World Health Organization; CRC, colorectal cancer.
Table 2
Polyp Characteristics of the Study Patients with SPS
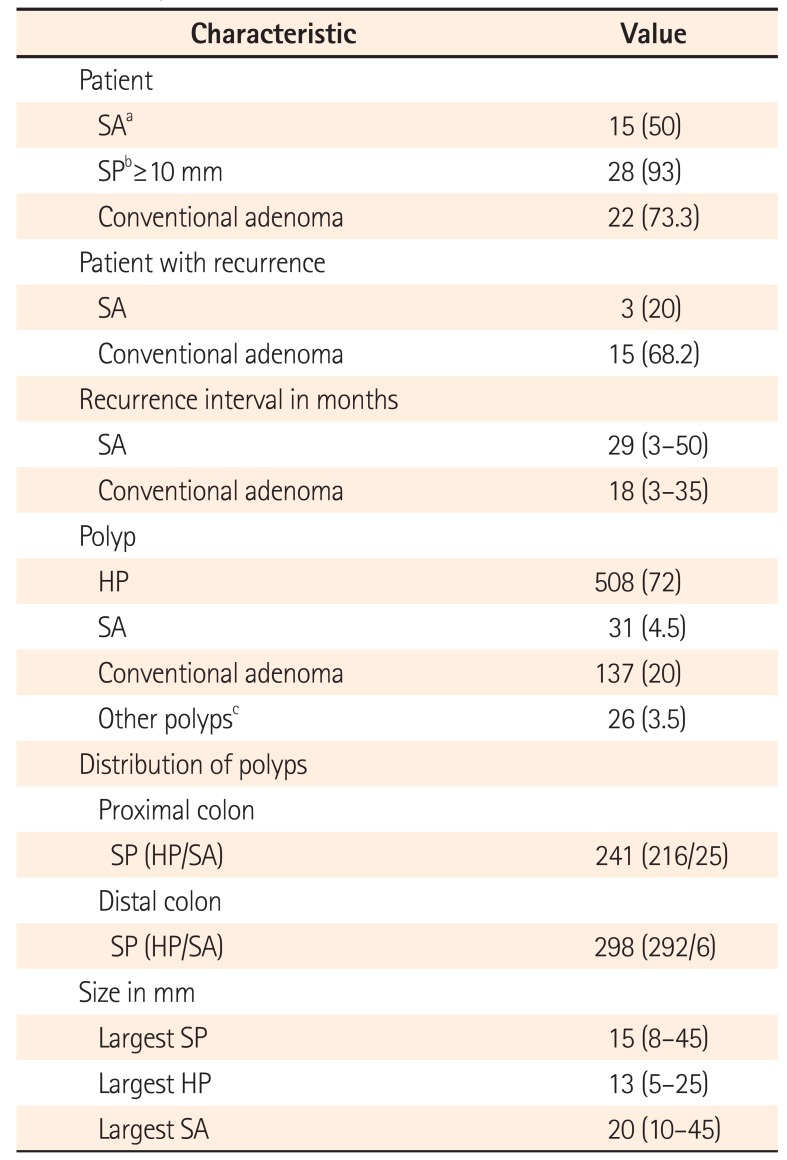
Values are presented as number (%) or median (range).
aSA includes sessile serrated adenoma and traditional serrated adenoma.
bSPs include hyperplastic polyp and serrated adenoma.
cOther polyps include inflammatory pseudopolyp, mucosal tag, and hamartomatous polyp.
SPS, serrated polyposis syndrome; SA, serrated adenoma; SP, serrated polyp; HP, hyperplastic polyp.
Table 3
Median Cumulative Number of Polyps per Person during Follow-Up
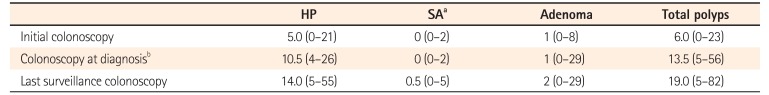
Table 4
Summary of Characteristics of Patients with Serrated (Hyperplastic) Polyposis in Recent Studies with More Than 10 Cases
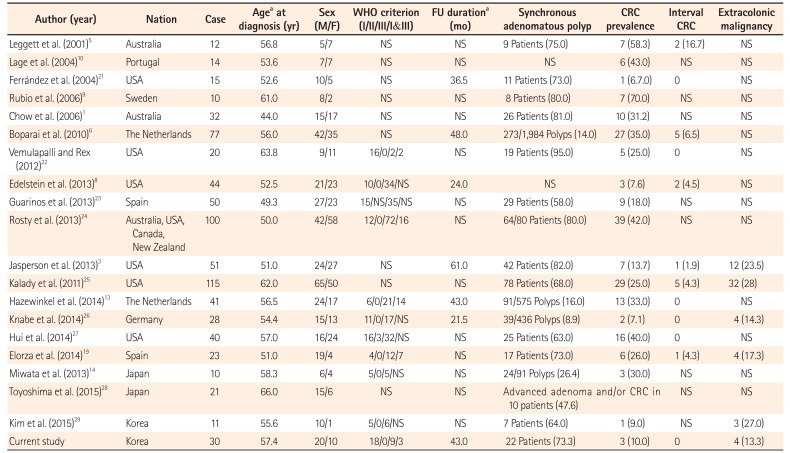
| Author (year) | Nation | Case | Agea at diagnosis (yr) | Sex (M/F) | WHO criterion (I/II/III/I&III) | FU durationa (mo) | Synchronous adenomatous polyp | CRC prevalence | Interval CRC | Extracolonic malignancy |
|---|---|---|---|---|---|---|---|---|---|---|
| Leggett et al. (2001)5 | Australia | 12 | 56.8 | 5/7 | NS | NS | 9 Patients (75.0) | 7 (58.3) | 2 (16.7) | NS |
| Lage et al. (2004)10 | Portugal | 14 | 53.6 | 7/7 | NS | NS | NS | 6 (43.0) | NS | NS |
| Ferrández et al. (2004)21 | USA | 15 | 52.6 | 10/5 | NS | 36.5 | 11 Patients (73.0) | 1 (6.7.0) | 0 | NS |
| Rubio et al. (2006)9 | Sweden | 10 | 61.0 | 8/2 | NS | NS | 8 Patients (80.0) | 7 (70.0) | NS | NS |
| Chow et al. (2006)1 | Australia | 32 | 44.0 | 15/17 | NS | NS | 26 Patients (81.0) | 10 (31.2) | NS | NS |
| Boparai et al. (2010)6 | The Netherlands | 77 | 56.0 | 42/35 | NS | 48 | 273/1,984 Polyps (14.0) | 27 (35.0) | 5 (6.5) | NS |
| Vemulapalli and Rex (2012)22 | USA | 20 | 63.8 | 9/11 | 16/0/2/2 | NS | 19 Patients (95.0) | 5 (25.0) | 0 | NS |
| Edelstein et al. (2013)8 | USA | 44 | 52.5 | 21/23 | 10/0/34/NS | 24.0 | NS | 3 (7.6) | 2 (4.5) | NS |
| Guarinos et al. (2013)23 | Spain | 50 | 49.3 | 27/23 | 15/NS/35/NS | NS | 29 Patients (58.0) | 9 (18.0) | NS | NS |
| Rosty et al. (2013)24 | Australia, USA, Canada, New Zealand | 100 | 50.0 | 42/58 | 12/0/72/16 | NS | 64/80 Patients (80.0) | 39 (42.0) | NS | NS |
| Jasperson et al. (2013)3 | USA | 51 | 51.0 | 24/27 | NS | 61.0 | 42 Patients (82.0) | 7 (13.7) | 1 (1.9) | 12 (23.5) |
| Kalady et al. (2011)25 | USA | 115 | 62.0 | 65/50 | NS | NS | 78 Patients (68.0) | 29 (25.0) | 5 (4.3) | 32 (28) |
| Hazewinkel et al. (2014)13 | The Netherlands | 41 | 56.5 | 24/17 | 6/0/21/14 | 43.0 | 91/575 Polyps (16.0) | 13 (33.0) | 0 | NS |
| Knabe et al. (2014)26 | Germany | 28 | 54.4 | 15/13 | 11/0/17/NS | 21.5 | 39/436 Polyps (8.9) | 2 (7.1) | 0 | 4 (14.3) |
| Hui et al. (2014)27 | USA | 40 | 57.0 | 16/24 | 16/3/32/NS | NS | 25 Patients (63.0) | 16 (40.0) | 0 | NS |
| Elorza et al. (2014)19 | Spain | 23 | 51.0 | 19/4 | 4/0/12/7 | NS | 17 Patients (73.0) | 6 (26.0) | 1 (4.3) | 4 (17.3) |
| Miwata et al. (2013)14 | Japan | 10 | 58.3 | 6/4 | 5/0/5/NS | NS | 24/91 Polyps (26.4) | 3 (30.0) | NS | NS |
| Toyoshima et al. (2015)28 | Japan | 21 | 66.0 | 15/6 | NS | NS | Advanced adenoma and/or CRC in 10 patients (47.6) | NS | NS | |
| Kim et al. (2015)29 | Korea | 11 | 55.6 | 10/1 | 5/0/6/NS | NS | 7 Patients (64.0) | 1 (9.0) | NS | 3 (27.0) |
| Current study | Korea | 30 | 57.4 | 20/10 | 18/0/9/3 | 43.0 | 22 Patients (73.3) | 3 (10.0) | 0 | 4 (13.3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download