Abstract
Background/Aims
Methods
Results
ACKNOWLEDGEMENTS
Notes
Financial support: The study was supported by Mochida Pharmaceutical Co., Ltd. Multimatrix mesalazine was kindly donated by Shire US Inc., Wayne, PA, USA. Mochida Pharmaceutical Co., Ltd. provided funding to support the provision of pH-dependent-release mesalazine (Zeria Pharmaceutical Co., Ltd., Tokyo, Japan).
Conflict of interest: Haruhiko Ogata has received consulting, grant, or lecture fees from Mochida Pharmaceutical, JIMRO, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Kyorin Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Eisai, Zeria Pharmaceutical, AbbVie G.K., EA Pharma, and Boston Scientific Japan K.K. Seiichi Mizushima and Atsushi Hagino are employees of Mochida Pharmaceutical. Toshifumi Hibi is editor-in-chief and has received consulting, grant, lecture, or manuscript preparation fees from Mochida Pharmaceutical, AbbVie G.K., EA Pharma, AstraZeneca K.K., JIMRO, Mitsubishi Tanabe Pharma, Eisai, Takeda Pharmaceutical, Zeria Pharmaceutical, Janssen Pharmaceutical K.K., Astellas Pharma, and Otsuka Pharmaceutical.
References
Fig. 1
Patient disposition. aMultiple options are allowed as reasons for discontinuation. FAS, full analysis set; UC-DAI, UC-Disease Activity Index; PPS, per protocol set.

Fig. 2
The proportion of patients who achieved remission, clinical remission, endoscopic remission, and improvement at the end of the treatment period in the per protocol set. The differences in each endpoint between the multimatrix mesalazine 4.8 g/day group and pH-dependent−release mesalazine 3.6 g/day group were 12.8% (two-sided 95% CI, 1.4% to 24.3%) for remission, 10.6% (two-sided 95% CI, −0.8% to 22.1%) for clinical remission, 5.4% (two-sided 95% CI, −3.5% to 14.2%) for endoscopic remission, and 9.0% (two-sided 95% CI, −2.7% to 20.8%) for improvement. aThe difference in the remission rate between the treatment groups was statistically significant.

Table 1
UC-Disease Activity Index
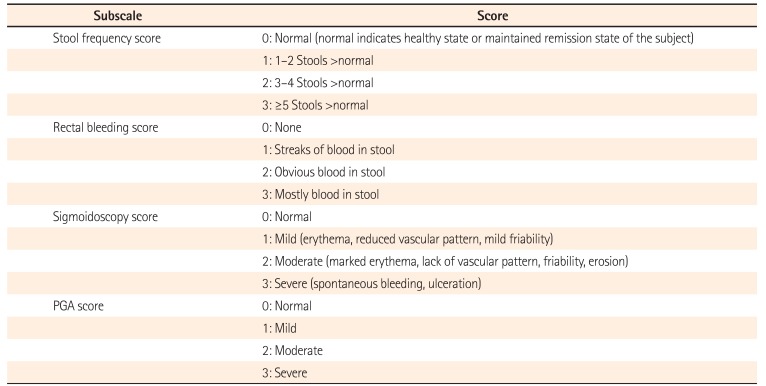
Table 2
Patient Demographics (Full Analysis Set)
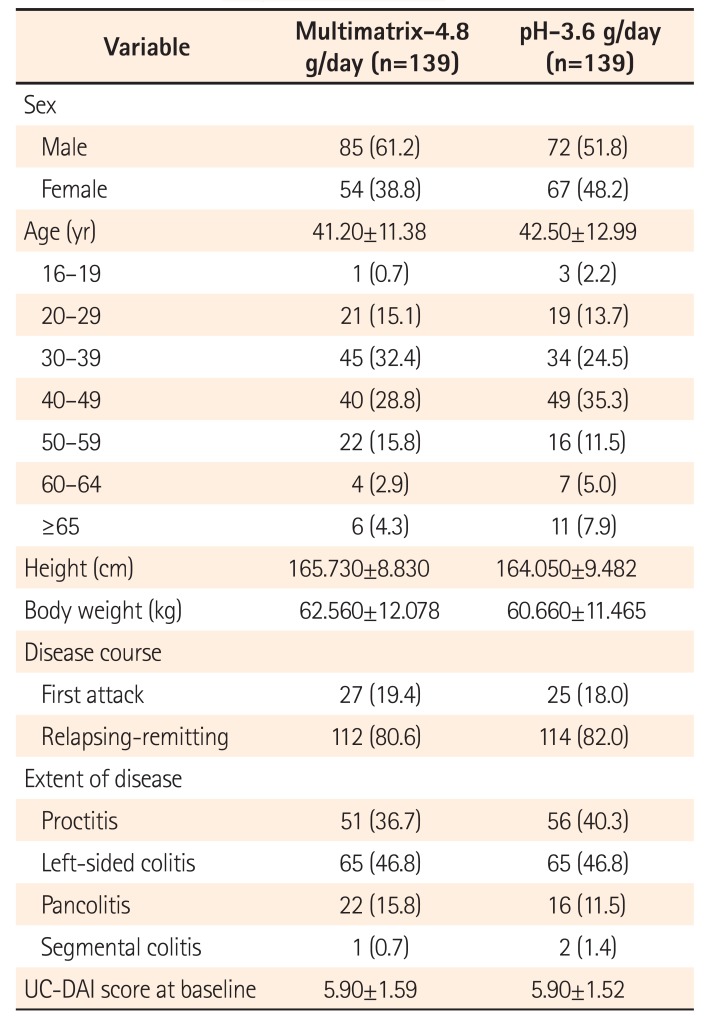
Table 3
Change in UC-Disease Activity Index Score at the End of Treatment
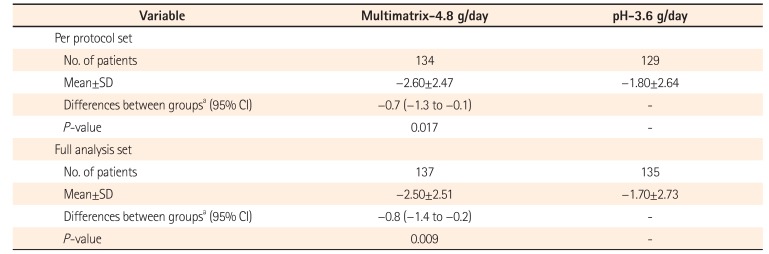
Table 4
Change in UC-Disease Activity Index Score Variables (Per Protocol Set)
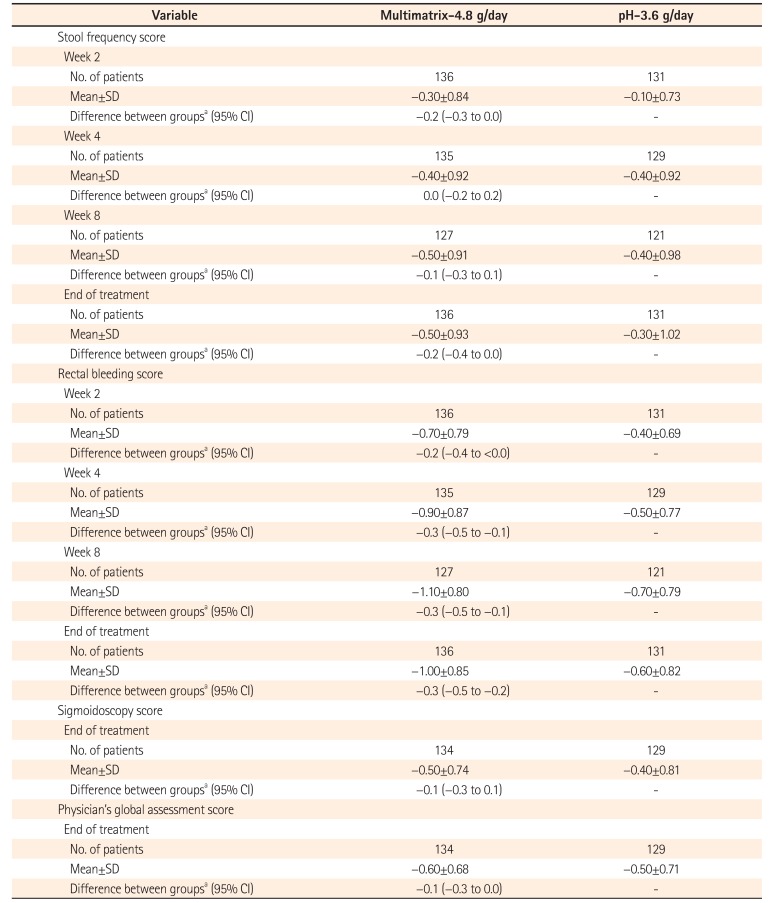
Table 5
Subgroup Analysis of Change in UC-Disease Activity Index Score (Per Protocol Set)
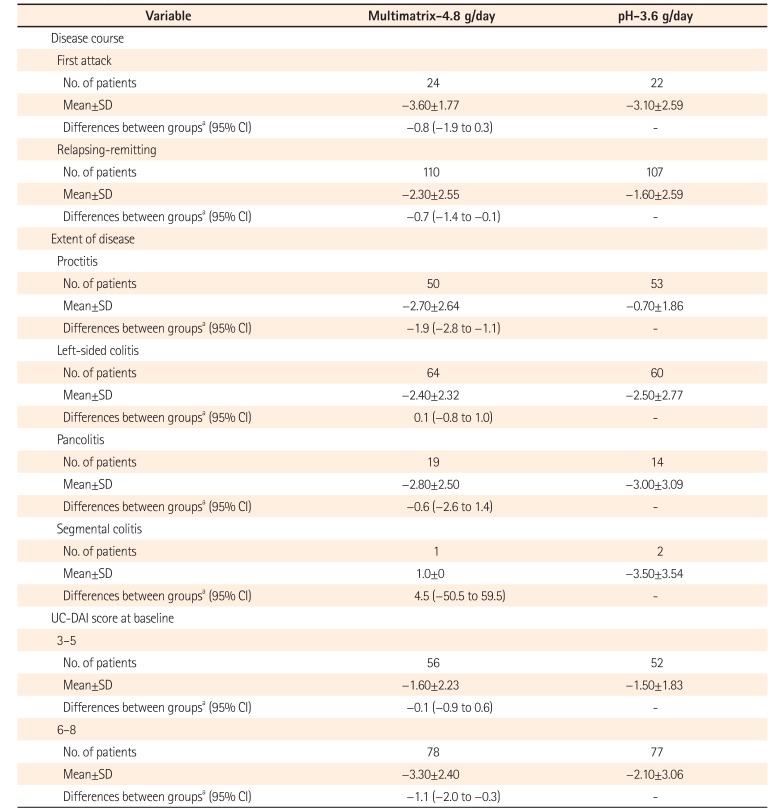
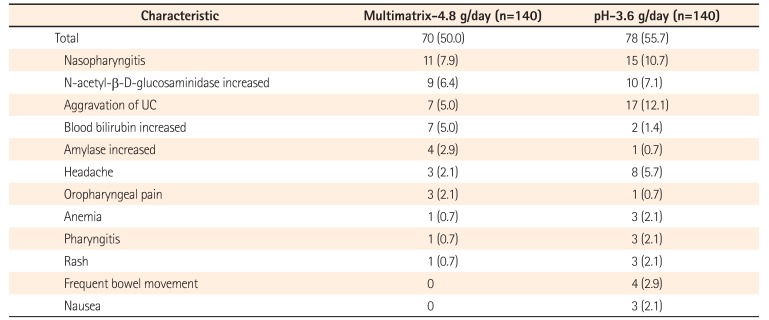
Table 6
Incidence of Adverse Events




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download