Abstract
Background/Aims
Recent genome-wide analyses have provided strong evidence concerning adverse events caused by thiopurine drugs such as azathioprine (AZA) and 6-mercaptopurine. The strong associations identified between NUDT15 p.Arg139Cys and thiopurine-induced leukopenia and severe hair loss have been studied and confirmed over the last 2 years. However, other coding variants, including NUDT15 p.Val18_Val19insGlyVal, NUDT15 p.Val18Ile, and FTO p.Ala134Thr, and a noncoding variation in RUNX1 (rs2834826) remain to be examined in detail in this respect. Therefore, we investigated the correlation between these adverse events and the 5 recently identified variants mentioned above among Japanese patients with inflammatory bowel diseases (IBD).
Methods
One hundred sixty thiopurine-treated patients with IBD were enrolled. Genotyping was performed using TaqMan SNP Genotyping Assays or Sanger sequencing.
Results
None of the 5 variants were associated with gastrointestinal intolerance to AZA. However, NUDT15 p.Arg139Cys was significantly associated with the interval between initiation and discontinuation of AZA among patients with gastrointestinal intolerance. This variant was strongly associated with early (<8 weeks) and late (≥8 weeks) leukopenia and severe hair loss. Moreover, it correlated with the interval between initiation of thiopurine therapy and leukopenia occurrence, and average thiopurine dose. NUDT15 p.Val18_Val19insGlyVal, NUDT15 p.Val18Ile, FTO p.Ala134Thr, and RUNX1 rs2834826 exhibited no significant relationship with the adverse events examined.
The thiopurine drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) have been used for many years for the treatment of numerous disorders, including UC, CD, acute lymphoblastic leukemia (ALL), and rheumatoid arthritis. In IBD (either UC or CD), thiopurines are used together with 5-aminosalicylic acid or the anti-tumor necrosis factor α antibodies infliximab and adalimumab to induce or maintain remission.12
Although thiopurine therapy has obvious clinical benefits in IBD, AZA and 6-MP occasionally induce severe adverse events such as leukopenia and alopecia. Thiopurine-induced severe leukopenia can result in infectious diseases with life-threatening consequences. Therefore, careful monitoring of white blood cell (WBC) count is required during treatment with these agents. Severe hair loss causes considerable stress to patients, and recovery may take more than 6 months following discontinuation of thiopurine therapy. These adverse events contribute to the interruption or discontinuation of thiopurine treatment. Therefore, genetic risk factors predictive of such thiopurine-induced effects have been studied in patients with IBD.3 Certain polymorphisms of thiopurine methyltransferase (TPMT) are well-characterized genetic markers of risk of thiopurine-induced adverse events, and TPMT genotyping prior to initiation of thiopurine treatment is recommended by the U.S. Food and Drug Administration. However, it is believed that other factors affecting the risk of such adverse events are present in the Japanese population, since TPMT risk allele frequencies in this country are lower than those in Western countries, and the incidence of adverse reactions to thiopurines is high in Japan, even among carriers of wild-type TPMT.345
In 2014, Yang et al.6 reported that a missense variant in exon 3 of the NUDT15 gene (p.Arg139Cys or R139C; single nucleotide polymorphism [SNP] ID: rs116855232) strongly correlates with thiopurine-induced leukopenia among Korean patients with CD. The high specificity and sensitivity (89.4% and 93.2%, respectively) of NUDT15 p.Arg139Cys recorded by these authors indicate that this variant, rather than TPMT polymorphisms, may be an effective genetic marker for predicting thiopurine-induced adverse events, at least in East Asian populations. To date, this strong association has been confirmed in numerous studies involving subjects with IBD or ALL.789101112131415161718 In addition, a further investigation identified 4 NUDT15 coding variants, including p.Arg139Cys, that influence both nucleotide diphosphatase activity and levels of thiopurine active metabolites, and found loss-of-function NUDT15 variants to be associated with thiopurine intolerance.13 Moreover, a more recent genome-wide association study conducted by Kim et al.19 found an FTO coding variant and a proximal region of RUNX1 to be associated with thiopurine-induced leukopenia among East Asian patients with IBD.
These findings suggest that the p.Arg139Cys coding variant of NUDT15 may be a highly reliable marker of thiopurine cytotoxicity risk, especially in Asian populations. However, in patients lacking a risk-associated TPMT variant and NUDT15 p.Arg139Cys, thiopurine cytotoxicity may be explained by the other abovementioned NUDT15 coding sequence variations or FTO or RUNX1 variants. Therefore, it is important to examine correlations between genes recently identified using genome-wide analysis and adverse clinical events.
Thus, in the present study, we investigated the associations between 3 coding variants in NUDT15 and thiopurine-induced adverse events among Japanese subjects. Furthermore, we examined potential relationships between single sequence variations in FTO and RUNX1 with such events in a Japanese population.
One hundred sixty Japanese patients with CD, UC, IBD unclassified, or intestinal Behçet disease having visited the Hyogo College of Medicine Hospital were included in this study. All patients, the characteristics of whom are shown in Table 1, were treated with AZA or 6-MP. This study was approved by the Ethical Review Board of Hyogo College of Medicine (approval number: Rin-hi-322), and written informed consent was obtained from all patients before their inclusion in this study.
Initial AZA and 6-MP doses of 25 to 50 and 10 to 20 mg/day, respectively, were administered. Generally, signs of adverse events were checked 2 to 3 weeks after treatment initiation, and when none were detected, AZA and 6-MP doses were raised to 50 to 100 and 30 to 50 mg/day, respectively. Nausea is an adverse event commonly associated with thiopurine therapy. Mild nausea symptoms are usually transient, but severe nausea can lead to discontinuation of thiopurine treatment.20 In this study, the group defined as having gastrointestinal (GI) intolerance to AZA included only those patients who discontinued AZA therapy because of GI symptoms such as severe nausea and epigastralgia. However, AZA-intolerant patients with associated GI symptoms who were successfully switched to 6-MP were not included in the GI intolerance group. We defined leukopenia as a WBC count <3,000/µL, and severe hair loss as objective hair loss that might necessitate the wearing of a wig and requiring a few months for recovery.7 Early or late leukopenia was defined as in previous studies (early leukopenia, within 8 weeks; late leukopenia, after 8 weeks).67
Genomic DNA was extracted from peripheral blood using a Blood & Cell Culture DNA Midi Kit (Qiagen, Hilden, Germany) or a BioRobot EZ1 (Qiagen) at our college. Of the 160 blood samples, 39 collected between June and November 2016 were sent to the LSI Medience Corporation (Tokyo, Japan) for DNA extraction and analysis.
Genotyping of NUDT15 p.Arg139Cys (rs116855232), FTO p.Ala134Thr (rs79206939), and a proximal region of RUNX1 (rs2834826) was performed using TaqMan SNP Genotyping Assays (assay IDs: C_154823200_10, C_102162425_10, and C_3266345_10, respectively) with TaqMan Genotyping Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR and allelic discrimination analysis were carried out using an ABI 7500 system (Applied Biosystems, Foster City, CA, USA). As PCR using probes for NUDT15 p.Arg139Cys is not effective for samples from individuals carrying NUDT15 p.Arg139His (rs147390019), genotyping of the latter was attempted by Sanger sequencing when the TaqMan SNP Assay for the former failed. The primer sequences used for Sanger sequencing of NUDT15 exon 3 were as follows: forward, AAGCAAATGCAAAGCATCAC; and reverse, GGCTGAAAGAGTGGGGGATA.13 The primers used to detect both NUDT15 p.Val18_Val19insGlyVal (rs554405994) and p.Val18Ile (rs186364861) in exon 1 using Sanger sequencing were as follows: forward, CAAAGCACAACTGTAAGCGACT; and reverse, GAAAGACCCAGCTAGCAAAGAC.13 The PCR products were purified using an ExoSAP-IT PCR Product Cleanup Kit (Affymetrix, Santa Clara, CA, USA), and sequencing was performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The sequencing products were purified with a BigDye XTerminator™ Purification Kit and analyzed on a 3130xl or 3500xl genetic analyzer (Applied Biosystems).
Categorical variables were compared using the chi-square test, or Fisher test when the expected value of any cell was less than 5. A Mann-Whitney or Kruskal-Wallis test was used to compare the difference in thiopurine dose or interval between each genotype. Statistical analysis was conducted using GraphPad Prism version 6.07 (GraphPad Software, La Jolla, CA, USA) and P-values <0.05 were considered statistically significant.
We recruited 160 patients with CD, UC, IBD unclassified, or intestinal Behçet disease in this study, the characteristics of whom are summarized in Table 1. The mean age of patients with leukopenia was significantly higher than that of those without leukopenia (P=0.0069). Although 6-MP-treated patients were more predisposed to leukopenia than AZA-treated patients, this is likely because physicians preferentially administer 6-MP, which exists as a powdered formulation suitable for small dose reductions, to thiopurine-treated patients whose WBC count declines. No significant differences were observed with respect to sex, body weight, disease type, use of co-medications such as 5-aminosalicylic acid, prednisolone, and anti-tumor necrosis factor α agents, initial thiopurine dose, or follow-up period.
Genotype frequencies of the 6 variants analyzed are shown in Table 2. Of the 160 subjects, 11 (6.9%), 2 (1.2%), and 9 (5.6%) were heterozygous for NUDT15 p.Val18_Val19insGly-Val, NUDT15 p.Val18Ile, and FTO p.Ala134Thr, respectively. None were homozygous for these variants. Wild-type, heterozygous, and homozygous variant NUDT15 p.Arg139Cys genotypes were carried by 117 (73.1%), 35 (21.9%), and 8 (5.0%) patients, respectively. For the rs2834826 noncoding sequence variation in RUNX1, 60 wild-type (37.5%), 80 heterozygous (50.0%), and 20 homozygous variant (12.5%) individuals were identified. The NUDT15 p.Arg139His variant was not detected in our study sample.
Of the 160 patients, 10 AZA- and 1 6-MP-treated patient experienced intolerable epigastralgia or nausea after initiation of therapy, and treatment was therefore discontinued. First, we investigated the correlation between GI intolerance to AZA and the coding variants of NUDT15. Nitroimidazole, which derives from AZA and causes nausea, is absent from 6-MP.21 Treatment with 6-MP may thus help to prevent or diminish nausea.22 Therefore, only AZA-treated patients were included in this analysis (Table 3).
The 4 nucleotide sequence variations leading to changes in the amino acid sequence of the NUDT15 protein are located in exon 1 (rs554405994 [p.Val18_Val19insGlyVal] and rs186364861 [p.Val18Ile]) and exon 3 (rs116855232 [p.Arg139Cys] and rs147390019 [p.Arg139His]).13 The 3 NUDT15 coding variants identified in our study group (p.Val18_Val19insGlyVal, p.Val18Ile, and p.Arg139Cys) were not significantly associated with GI intolerance to AZA (Table 3). However, comparing NUDT15 p.Arg139Cys genotypes with respect to the interval between initiation and discontinuation of AZA therapy revealed that patients carrying the C/T or T/T genotype discontinued AZA significantly earlier than those with the wild-type C/C genotype (Fig. 1). These data suggest that although GI intolerance to AZA occurred regardless of NUDT15 genotype, p.Arg139Cys risk allele carriers discontinued AZA earlier than wild-type carriers owing to differing symptom severity.
The NUDT15 p.Arg139Cys variant is in current use as a reliable marker of risk of thiopurine-induced leukopenia and severe hair loss. However, it remains unclear whether other coding variants also cause these adverse events in patients with IBD. Therefore, we next analyzed the correlation between 3 NUDT15 coding variants and these events. The 11 subjects who, due to GI intolerance, discontinued AZA (Table 3) or 6-MP therapy 1 to 9 weeks after its initiation were excluded from this analysis, since this time period was insufficient to evaluate leukopenia or severe hair loss. One hundred forty-nine subjects were therefore assessed, and the results are presented in Table 4. The frequencies of p.Val18_Val19insGlyVal, p.Val18Ile, and p.Arg139Cys alleles were similar to those shown in Table 3. The coding variants in exon 1 of NUDT15 (p.Val18_Val19insGlyVal and p.Val18Ile) were not associated with leukopenia or severe hair loss. In contrast, strong associations with that in exon 3 (p.Arg139Cys) were evident. The P-value for the relationship between p.Val18_Val19insGlyVal genotype and leukopenia (no leukopenia versus combined early and late leukopenia) was 0.0512. However, this variant is in high linkage disequilibrium with p.Arg139Cys;13 therefore, the effect of p.Val18_Val19insGlyVal alone may not be strong.
We identified 2 subjects heterozygous for the p.Val18Ile variant, both of whom exhibited late leukopenia. However, this relationship was not statistically significant (P=0.1029) owing to the relatively small sample size. These 2 patients developed leukopenia approximately 3 years after initiation of AZA treatment, and quickly recovered after interruption of this therapy, without granulocyte-colony stimulating factor administration.
Further analysis of p.Arg139Cys genotypes was performed, the results of which are shown in Fig. 2. The mean interval from initiation of thiopurine therapy to leukopenia among subjects carrying the C/C, C/T, and T/T genotype was 19.81, 13.67, and 1.01 months, respectively (P=0.0009) (Fig. 2A). None of the patients with the T/T genotype were able to continue thiopurine therapy for more than 9 weeks due to GI intolerance, leukopenia, or severe hair loss. To compare the average thiopurine dose administered between each genotype, we considered only subjects having continued thiopurine therapy for more than 1 year. Carriers of the C/T genotype tended to require frequent reductions in thiopurine dose during the first year of therapy due to leukopenia. As a result, average thiopurine doses given to patients with the C/T genotype were significantly lower than those administered to C/C genotype carriers (0.77 and 1.01 mg/kg/day, respectively; P=0.0296) (Fig. 2B).
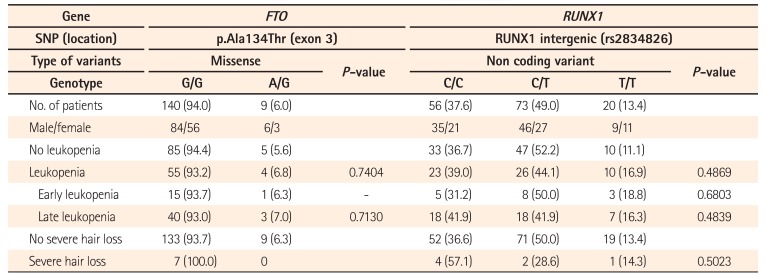
Many FTO and RUNX1 loci have been associated with thiopurine-induced leukopenia in the East Asian population.19 In particular, a non-synonymous coding sequence variation in FTO (p.Ala134Thr, rs79206939) has been shown to reduce FTO activity. Consistent with these results, FTO−/− and FTO+/− mice exhibit increased sensitivity to thiopurine.19 Therefore, we investigated the association of the FTO p.Ala134Thr variation and a noncoding variant in the proximal region of RUNX1 (rs2834826) with thiopurine-induced leukopenia and severe hair loss among Japanese patients with IBD.
Nine subjects were heterozygous for the A risk allele of FTO p.Ala134Thr. Of these, 4 developed leukopenia, but this association was not statistically significant (P=0.7404). Moreover, none of the 9 patients exhibited severe hair loss. RUNX1 rs2834826 was not associated with leukopenia or severe hair loss (P=0.4869 and P=0.5023, respectively) (Table 5).
AZA and 6-MP have been widely used as immunomodulators and chemotherapeutic agents for the treatment of various diseases. At our hospital, more than 100 patients with IBD commence thiopurine therapy each year, and this number is increasing due to the growing incidence of IBD in Japan. Every year, several patients present with early leukopenia or severe hair loss after initiating thiopurine treatment. To date, for such patients carrying the wild-type TPMT genotype, the cause of these adverse events could not be determined.
Discovery of the association between NUDT15 p.Arg139Cys and thiopurine-induced leukopenia among Korean CD patients may enable unprecedented progress in the management of adverse thiopurine effects in the East Asian population.6 Importantly, many investigators have been able to replicate these results in studies of thiopurine-treated patients with IBD or ALL.789101112131415161718 In the current study, we successfully established a significant association between NUDT15 p.Arg139Cys and thiopurine-induced leukopenia and severe hair loss.
We obtained evidence that this variant correlates with the time interval between AZA initiation and GI intolerance to this drug (Fig. 1). The abnormal accumulation of thiopurine metabolites in patients carrying p.Arg139Cys may accelerate development of strong nausea. However, our results in this respect may be limited by certain arbitrary aspects of the analysis, given the subjectivity of nausea and that the decision to discontinue thiopurine therapy is dependent on physicians and patients.
As evidence of the role of NUDT15 in thiopurine treatment accumulates, the demand for NUDT15 genotyping prior to such therapy is increasing in Japan. Although these replicable results should be translated into clinical practice, further evaluation of NUDT15 variants is necessary before clinical application. Consequently, we investigated three coding sequence variations in NUDT15 identified by Moriyama et al.13 No association between the p.Val18_Val19insGlyVal and p.Val18Ile variants and leukopenia or severe hair loss was evident in our study. However, it should be noted that these adverse events did occur more frequently among subjects heterozygous for these coding variants (Table 4); therefore, it is likely that they are significantly more common among homozygous risk allele carriers, as observed in relation to the p.Arg139Cys mutation. The strong linkage disequilibrium between p.Val18_Val19insGlyVal and p.Arg139Cys should be taken into account however.13 These coding variants result in 74.4% to 100% loss of NUDT15 enzymatic activity.13 Therefore, carriers of the exon 1 variation may need to be followed-up carefully to monitor for signs of leukopenia. Future studies are expected to shed light on the importance of p.Val18_Val19insGlyVal and p.Val18Ile as predictive markers of thiopurine-induced adverse events.
In our study sample, 8 subjects homozygous for the T allele of NUDT15 p.Arg139Cys were identified. One of these individuals discontinued AZA therapy within 7 days following its initiation due to intolerable GI symptoms (Table 3, Fig. 1). Six of the 8 subjects were able to continue AZA treatment for more than 2 weeks, but discontinued it because of early or late leukopenia, the latter being detected at 9 weeks (Table 4). All of the homozygous T/T subjects with leukopenia exhibited grade 4 leukopenia (defined as a WBC <1,000 cells/µL) and required both granulocyte-colony stimulating factor and antibiotics. Only 1 patient carrying this genotype did not develop leukopenia. However, all 7 patients who continued thiopurine therapy for more than 2 weeks experienced severe hair loss. Therefore, administration of thiopurines to patients with the NUDT15 p.Arg139Cys T/T genotype caused drastic adverse events. We currently do not use thiopurine therapy for patients with IBD and this genotype because it is contraindicated. It remains unclear whether an extremely low dose of 6-MP would be tolerable or clinically efficacious for Japanese carriers of this genotype suffering IBD.
The physiological function of NUDT15 is considered to be the hydrolysis of 8-oxo-dGTP generated from reactive oxygen species.23 Recent studies indicate that NUDT15 preferentially inactivates thiopurine metabolites over 8-oxo-dGTP,24 converting those such as TGTP and TdGTP to TGMP and TdGMP, respectively.13 NUDT15 is a negative regulator that decreases levels of bioactive thiopurine metabolites, the cytotoxicity of which is therefore increased by loss-of-function variants. Our data obtained from analyzing adverse events among thiopurine-treated patients indicate that the NUDT15 Arg139Cys site may be more critical to thiopurine metabolism than those loci affected by the other coding variants examined. The precise molecular mechanisms by which the p.Arg139Cys mutation leads to thiopurine-induced cell death remain unclear.
The site of the Arg139Cys mutation is reported to be within the TGTP-binding pocket of the NUDT15 protein. Substitution of arginine with cysteine at this position may affect the enzyme's structure, and thereby, reduce cellular TGTP hydrolysis.24 One study has shown that NUDT15 proteins harboring the Arg139Cys mutation demonstrate enzyme activity comparable to that of the wild-type form, but are degraded rapidly in cells.25 Therefore, NUDT15 protein level may be implicated in the cytotoxicity of and sensitivity to thiopurines. The development of biochemical tests of NUDT15 enzymatic activity would be beneficial.
Sequence variations in the genes FTO and RUNX1 identified using a genome-wide association study are also promising candidates as predictive markers of thiopurine cytotoxicity.19 However, we could not verify the association between thiopurine-induced adverse events and FTO p.Ala134Thr and a noncoding variant in the proximal region of RUNX1 among Japanese patients with IBD. This may be due to differences between Korean and Japanese patients with this condition in terms of the thiopurine doses administered. We usually initiate AZA and 6-MP treatment at relatively low doses (25 and 10–20 mg/day, respectively), and sometimes gradually increase the dose while paying attention to the development of adverse events such as GI symptoms and leukopenia. For Japanese patients with IBD, even low doses of thiopurines are clinically effective and result in therapeutic concentrations of the thiopurine metabolite 6-thioguanine nucleotide;2627 therefore, the AZA maintenance dose of 25 to 100 mg/day (equivalent to 0.5–2.0 mg/kg/day for a patient weighing 50 kg) is commonly used, as shown in Fig. 2B. We did not identify any homozygous FTO p.Ala134Thr risk allele carriers in this study, who are expected to exhibit more prominent effects of thiopurine exposure. As our sample size was not large enough, further analysis will be required to elucidate the association between these genes and thiopurine-induced adverse events in the Japanese population.
In summary, we verified the strong association between NUDT15 p.Arg139Cys and thiopurine-induced early and late leukopenia and severe hair loss among Japanese patients with IBD. However, NUDT15 p.Val18_Val19insGlyVal, NUDT15 p.Val18Ile, FTO p.Ala134Thr, and a noncoding variant in the proximal region of RUNX1 (rs2834826) were not associated with these adverse events. Thus, NUDT15 p.Arg139Cys genotyping should currently be prioritized for the prediction of thiopurine-induced adverse events among Japanese patients with IBD, and its application in precision medicine should be considered in the future.
Notes
References
1. Yamada S, Yoshino T, Matsuura M, et al. Efficacy and safety of long-term thiopurine maintenance treatment in Japanese patients with ulcerative colitis. Intest Res. 2015; 13:250–258. PMID: 26131000.

2. Yoshino T, Matsuura M, Minami N, et al. Efficacy of thiopurines in biologic-naïve Japanese patients with Crohn's disease: a single-center experience. Intest Res. 2015; 13:266–273. PMID: 26131002.

3. Roberts RL, Barclay ML. Update on thiopurine pharmacogenetics in inflammatory bowel disease. Pharmacogenomics. 2015; 16:891–903. PMID: 26067482.

4. Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009; 24:1258–1264. PMID: 19682195.

5. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK. IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015; 13:193–207. PMID: 26130993.

6. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020. PMID: 25108385.

7. Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016; 16:280–285. PMID: 26076924.

8. Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016; 51:22–29. PMID: 26590936.

9. Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015; 33:1235–1242. PMID: 25624441.

10. Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. 2015; 171:109–115. PMID: 26033531.

11. Lee YJ, Hwang EH, Park JH, Shin JH, Kang B, Kim SY. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2016; 28:475–478. PMID: 26735160.

12. Liang DC, Yang CP, Liu HC, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016; 16:536–539. PMID: 26503813.

13. Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016; 48:367–373. PMID: 26878724.

14. Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate-linked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017; 32:620–624. PMID: 27416873.

15. Suzuki H, Fukushima H, Suzuki R, et al. Genotyping NUDT15 can predict the dose reduction of 6-MP for children with acute lymphoblastic leukemia especially at a preschool age. J Hum Genet. 2016; 61:797–801. PMID: 27193222.

16. Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016; 44:967–975. PMID: 27604507.

17. Zgheib NK, Akika R, Mahfouz R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children's Cancer Center of Lebanon. Pediatr Blood Cancer. 2017; 64:146–150. PMID: 27577869.

18. Chiengthong K, Ittiwut C, Muensri S, et al. NUDT15 c.415C>T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. 2016; 101:e24–e26. DOI: 10.3324/haematol.2015.134775.PMID: 26405151.

19. Kim HS, Cheon JH, Jung ES, et al. A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD [published online ahead of print August 24, 2016]. Gut. 2016; DOI: 10.1136/gutjnl-2016-311921.
20. Tanis AA. Azathioprine in inflammatory bowel disease, a safe alternative? Mediators Inflamm. 1998; 7:141–144. PMID: 9705598.

21. Roberts RL, Barclay ML. Current relevance of pharmacogenetics in immunomodulation treatment for Crohn's disease. J Gastroenterol Hepatol. 2012; 27:1546–1554. PMID: 22741564.

22. Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott ID. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 27:220–227. PMID: 17988235.

23. Takagi Y, Setoyama D, Ito R, Kamiya H, Yamagata Y, Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J Biol Chem. 2012; 287:21541–21549. PMID: 22556419.

24. Carter M, Jemth AS, Hagenkort A, et al. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat Commun. 2015; 6:7871. PMID: 26238318.

25. Valerie NC, Hagenkort A, Page BD, et al. NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 2016; 76:5501–5511. PMID: 27530327.

26. Andoh A, Tsujikawa T, Ban H, et al. Monitoring 6-thioguanine nucleotide concentrations in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008; 23:1373–1377. PMID: 18662197.

27. Komiyama T, Yajima T, Kubota R, et al. Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J Crohns Colitis. 2008; 2:315–321. PMID: 21172230.

Fig. 1
Interval from initiation to discontinuation of azathioprine among patients with gastrointestinal intolerance.

Fig. 2
(A) Interval from initiation of thiopurine treatment to leukopenia development according to NUDT15 p.Arg139Cys genotype. (B) Association between NUDT15 p.Arg139Cys genotype and thiopurine dose tolerated.

Table 1
Baseline Characteristics of Patients
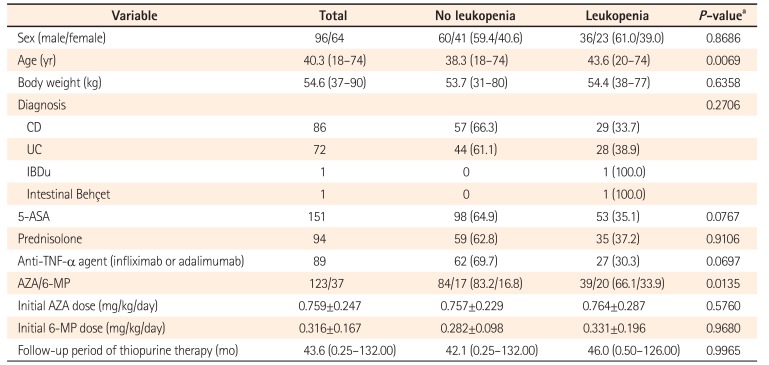
Table 2
Genotype Frequencies in Variants of NUDT15, FTO, and RUNX1
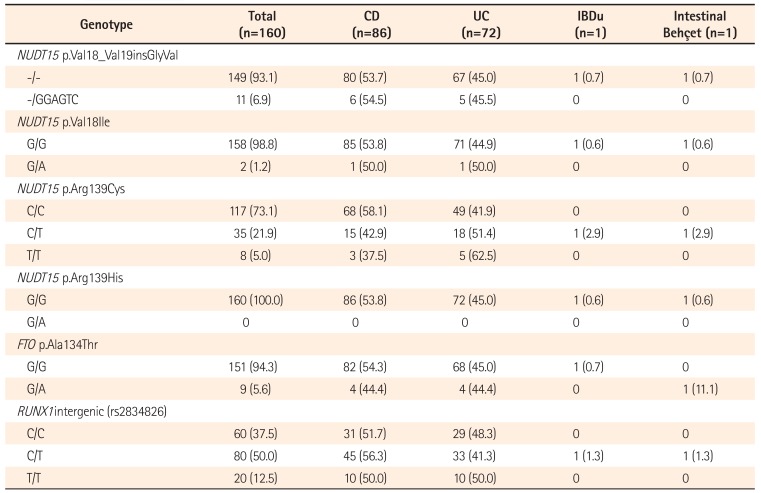
Table 3
No Correlation between NUDT15 Genotypes and the Incidence of GI Intolerance to Azathioprine
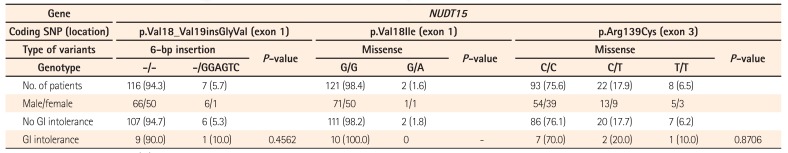
Table 4
Associations between NUDT15 Genotype and Leukopenia and Severe Hair Loss
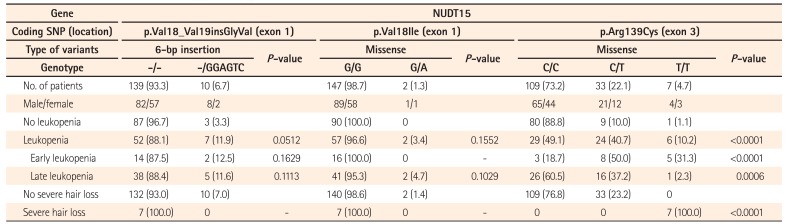
Table 5
No association of FTO and RUNX1 with Leukopenia and Severe Hair Loss in a Japanese Population




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download