This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
This study aimed to determine the effectiveness and safety of a newly developed endovenous radiofrequency (RF) catheter compared with that of the existing RF catheter in a canine model.
Methods
Seven dogs underwent ablation using 1 control catheter (ClosureFAST, CF; Covidien) and 1 experimental catheter (VENISTAR, VS; STARmed Co., Ltd.) in the femoral and cephalic veins. The ablated vein was evaluated macroscopically (2,3,5-triphenyltetrazolium chloride staining, TTC), microscopically (hematoxylin and eosin staining), and ultrasonographically. Vessel injury score was used to evaluate the ablating effect objectively. Veins from 1 dog were evaluated on the day of ablation, while in the remaining 6 dogs, the ablated veins were evaluated 2 weeks later.
Results
A total of 23 veins (CF, 11 veins; VS, 12 veins) were ablated in 7 dogs. Non–TTC-stained vein wall areas were identified in all ablated veins. No significant difference was observed in the mean vessel injury score (2.54 ± 1.16 vs. 2.42 ± 1.13, P = 0.656) and the mean vessel wall thickness (0.32 ± 0.03 mm vs. 0.31 ± 0.05 mm, P = 0.212) between CF and VS. There was no blood flow in all veins ablated with VS, whereas there was remaining blood flow in 1 vein ablated with CF. Perivenous complication was not observed.
Conclusion
Endovenous RF ablation using a newly developed VS RF catheter seems to provide comparable occlusion rate and degree of vein wall injury without perivenous adverse events compared to the most commonly used RF catheter (CF).
Go to :

Keywords: Canine model, Endovenous ablation, New electrode, Radiofrequency
INTRODUCTION
Endovenous radiofrequency ablation (RFA), which is responsible for 1 axis of chronic venous insufficiency treatment, uses a radiofrequency (RF) signal. RF waves are electromagnetic energy with a frequency of 300 kHz to 1 MHz, and when it comes in contact with tissue, vibration or friction of the ions converts their mechanical energy into thermal energy. This hyperthermic process causes cell damage to the venous wall, alters or destroys tissue structures, and causes fibrotic occlusion of incompetent veins, which finally atrophy [
12].
VNUS Closure (VNUS Medical Technologies Inc.) was first introduced as an endovenous RF device, but it has several drawbacks. The procedure took a long time, and if a clot was generated at the level of the electrode, the catheter had to be removed to clean the clot. To solve this problem, ClosureFAST (CF; Covidien), an improved device that enables segmental ablation, became available in 2007 [
12].
Although CF is still mainly used for endovenous RFA, complications such as skin injury may be a particular risk for CF, as opposed to other RF procedures, because of its direct heating effect, 120-℃ operating temperature, and slower cooling rate [
2]. Therefore, we assumed that if a device is available that keeps the catheter temperature low during the ablation process without generating heat from the catheter itself, complications related to thermal damage would be reduced.
A new bipolar electrode, VENISTAR (VS; STARmed Co., Ltd.), that performs ablation with a mechanism different from that of CF was developed. This mechanism is similar to that of Celon RF-induced thermal therapy (RFiTT; Olympus), which achieves ablation of the venous wall using frictional heat through ionic oscillation. In addition, an internal cooling system was applied inside the catheter, and a segmental ablation method was applied rather than a pullback method.
In this animal study, a newly developed RF electrode was inserted into the vein of the canine model and RF energy was delivered. The ablation pattern in the treatment site was analyzed and compared with that from the existing RF catheter (CF) to evaluate the effectiveness and safety of the new RF electrode.
Go to :

METHODS
Animal models
All animal experimental procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Cardiovascular Product Evaluation Center, Yonsei University (No. CPEC-IACUC-181005).
Seven healthy male mongrel dogs weighing 30 ± 5 kg were enrolled in the present study. The dogs were acclimated for 7 days before the experiments. Each dog was bred in a separate stainless-steel breeding box and was maintained at an environmental temperature of 20–24 ℃, relative humidity of 30%–70%, and a 12/12-hour light-dark cycle.
After induction of anesthesia through intramuscular administration of atropine 0.02–0.04 mg/kg (Jeil Pharmaceutical Co., Ltd.), Domitor 0.1 µg/kg (Elanco), Zoletil 10 mg/kg (Virbac), Rumpun 0.1–1.0 mg/kg (Bayer), Alfaxan 5.0 mg/kg (Jurox), and tramadol 1.0–3.0 mg/kg (Hanall Biopharma), respiratory anesthesia was maintained at 5–10 mL/kg/min with Forane (JW Pharmaceutical) and an O2 in a ratio of 1–2:1 using Primus (Dräger).
The experiment was carried out by inserting 1 control device (CF) and 1 experimental device (VS) for each dog into the femoral and cephalic veins and removing the catheter after ablation. Of the 7 dogs, 1 was sacrificed on the same day of the procedure, and the other 6 dogs were followed up for 2 weeks, with the experiment terminated following autopsy (
Table 1).
Table 1
Summary of the canine models and allocation of RF catheters

Ablation procedure
The animals were draped in a sterile fashion, and the skin was prepped with povidone-iodine and alcohol. Following exposure of the femoral and cephalic veins, a 7-French (Fr) sheath (Sungwon Medical Co., Ltd.) was inserted, and 200 IU/kg of heparin (JW Pharmaceutical) was injected into the sheath.
A 7-cm-long ablation catheter (VS or CF) for segmental ablation was inserted through the sheath and advanced to the treatment site. Proper positioning of the catheter was confirmed through ultrasonography (US; HD15, Philips) using a specialized transducer (L15-7io, Philips).
The veins of 1 dog to be sacrificed on the day of the procedure were ablated without tumescent injection of normal saline. For the remaining dogs, a sufficient amount of normal saline was injected around the target veins to be subjected to ablation.
When using the VS, an RF generator (VVR Generator, STARmed) connected to the ablation catheter was operated, and ablation was performed at 30 W for 25 seconds. The RF power was fixed at 30 W for all experiments in this study. When using the CF, segmental energy at 120 ℃ was delivered in 20-second cycles.
In cases of cephalic vein, ablation was performed twice at the proximal segment, the catheter was then withdrawn to the next segment (middle) through the graduation mark on the catheter, and then ablation was performed once. Subsequently, the catheter was withdrawn to the distal side of the target vein, and ablation was performed once again. Four ablations were performed in the cephalic vein. For femoral vein, ablations at the proximal and middle segments were performed thrice. However, the procedure was performed by adjusting the number of ablations considering the length of the target vein for each subject.
At the end of the procedure, the 7-Fr sheath was removed and the vein was repaired. The subcutaneous tissue and skin were then sutured. After the procedure, cephradine 100 mg/mL (Panzedin, Hankook Korus Pharm Co., Ltd.) was injected intramuscularly for 6 days.
Assessment
Gross and ultrasonography findings
For the 6 dogs that were followed up, the ablated vein was visually and ultrasonographically inspected for the presence of hematoma, swelling, or abscess formation during and at the end of follow-up. Whether blood flow remained in the ablated vein was also evaluated using US at the end of the follow-up period.
To evaluate whether there was a difference in the thickness of the vessel wall between the VS and CF after ablation, the thickness of the vessel wall was measured using US immediately before autopsy.
Histology
The ablated veins harvested after autopsy were irrigated with normal saline. They were immersed and shaded in a 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma) solution and incubated for 1 hour at 40 ℃. After the staining was completed, the veins were sectioned longitudinally and completely unfolded. The exposed ablated site was macroscopically checked, and photographs were taken.
TTC-stained femoral/cephalic veins were fixed in 10% neutral-buffered formalin. Each fixed tissue was rinsed in tap water for 24 hours to completely remove the fixative from the tissue. For tissue dehydration, the tissue was gradually dehydrated using high-concentration ethanol of 70%–100%, and then a paraffin block was produced by clearing with xylene. The prepared block was cut to a thickness of 5 µm using a microtome to prepare slides. The slides were stained with H&E for microscopic evaluation.
Verifying the nonstained area in the vein subjected to TTC staining identified the surviving and damaged areas in the venous endothelium, making it easier to select the area to be examined under the microscope. The part that was not stained with TTC was assessed as the part where vein injury occurred through ablation.
The vessel injury score analyzed based on H&E staining was also used to objectively evaluate the ablating effect. Vessel injury scores were measured at 3 sites per harvested ablated vein. After scanning the entire tissue made of slides with a scanner, the damaged area was visually checked. This method was applied by modifying that of a previous study [
3]. The criteria were assigned according to injury severity from 1 (least injury) to 4 (most injury): 1, endothelial cell coverage; 2, medial smooth muscle cell loss; 3, internal and external elastic lamina disruption; and 4, adventitia disruption. Scoring was comprehensively performed by a pathologist through evaluating the damaged area that each criterion had inflicted on the tissue.
Statistical analysis
The results of this experiment were compared by calculating the mean and standard deviations. Student t-test was used to compare differences in vessel injury score and vessel wall thickness. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 23 (IBM Corp.).
Go to :

RESULTS
A total of 23 veins (CF, 11 veins; VS, 12 veins) were ablated in 7 dogs. In 1 dog ablated without tumescent injection and sacrificed on the day of the procedure, 2 veins each were ablated with CF and VS. In 6 dogs, ablation was performed following tumescent injection in 9 veins using CF and 10 veins using VS.
During the ablation of 1 vein using VS catheter without tumescent injection, the wattage dropped before reaching 25 seconds, and the experimenter encountered resistance while withdrawing the catheter.
As a result of examining the injured vein through TTC staining, most of the ablated vein walls showed white, unstained areas, while a small portion of the ablated vein walls showed red, stained areas in both the CF and VS groups (
Fig. 1).
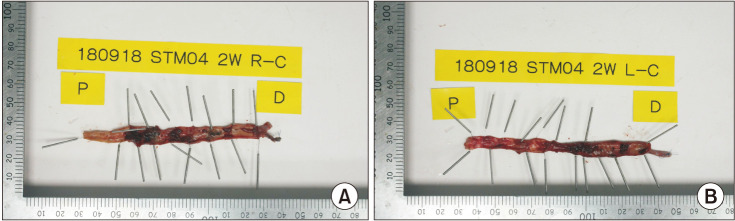 | Fig. 12,3,5-Triphenyltetrazolium chloride staining (TTC) stained ablated veins (follow-up day 14). Histologic specimen of cephalic veins of both sides in the same subject (the 4th dog) with (A) ClosureFAST (Covidien) and (B) VENISTAR (STARmed Co., Ltd.) shows TTC-unstained area suggesting loss of viability.
|
Microscopic evaluation through H&E staining showed varying degrees of vein wall injury at 69 sites in all ablated veins using CF and VS (
Fig. 2).
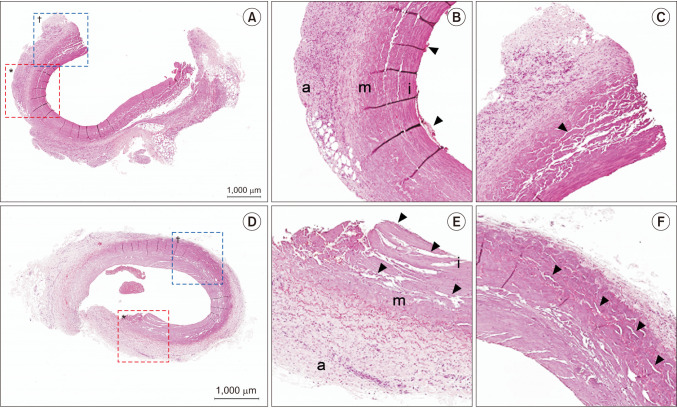 | Fig. 2H&E stained photomicrographs of damaged veins ablated with CF (A–C) and VS (D–F). (A) The entire cross section of cephalic vein in the 6th dog ablated with CF. (B) Enlarged image of the red dotted box in panel A. Destruction of endothelial cell layer with some blood cells (arrowheads) adjacent to the injured endothelial layer. (C) Enlarged image of the blue dotted box in panel A. The elastic fibers are not observed and clefts (arrowhead) are noted in the tunica media. (D) The entire cross section of cephalic vein in the 7th dog ablated with VS. (E) Enlarged image of the red dotted box in panel D. Destruction of endothelial cell layer and disintegration with lots of clefts in the tunica intima and media (arrowheads). (F) Enlarged image of the blue dotted box in panel D. Clefts noted in the tunica adventitia (arrowheads). a, tunica adventitia; m, tunica media; i, tunica intima; CF, ClosureFAST (Covidien); VS, VENISTAR (STARmed Co., Ltd.).
|
As a result of H&E staining, the mean vessel injury score was 2.54 ± 1.16 in CF in the control group and 2.42 ± 1.13 in VS in the experimental group (
Fig. 3A). No significant difference was observed between the 2 groups (P = 0.656). In addition, a vessel injury score of 4 was observed in 7 out of 33 sites (21.2%) in the CF group and 4 out of 36 sites (11.1%) in the VS group.
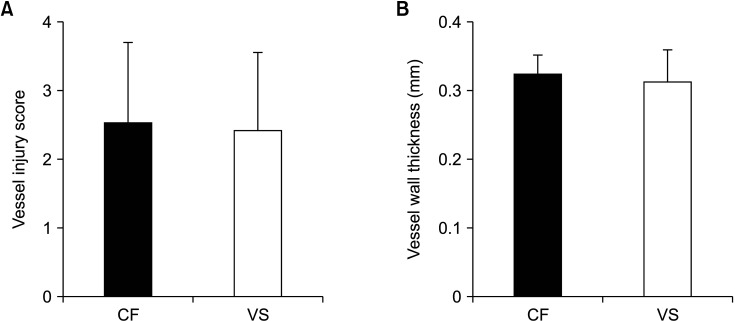 | Fig. 3Measurement of vessel injury score (A) and vessel wall thickness (B). CF, ClosureFAST (Covidien); VS, VENISTAR (STARmed Co., Ltd.).
|
Vessel wall thickness using US immediately before autopsy showed no significant differences between the CF and VS groups (0.32 ± 0.03 mm
vs. 0.31 ± 0.05 mm, respectively; P = 0.212) (
Fig. 3B).
Hematoma, swelling, or abscess was not observed visually or ultrasonographically for 14 days after the procedure. At the end of follow-up, blood flow remained in 1 vein (the 6th dog, left femoral vein) out of 9 veins (11.1%) ablated with CF on US evaluation. However, blood flow was not observed in all 10 veins (0%) ablated with VS on US evaluation.
Go to :

DISCUSSION
During endovenous RFA, sufficient energy should be provided to make a controllable and transmural heating of the venous wall; enough to induce collagen contraction and vein wall destruction [
1]. However, it is also important to minimize the degree of perivascular heating for safety. Unlike CF, which generates heat via RF energy heating of the integrated treatment coil element at the catheter, a newly developed RF catheter (VS) applies RF power to agitate tissue ions as they attempt to follow the changes in alternating current direction. The agitation of tissue ions creates frictional heat around the electrode.
VS obtains venous closure by applying an impedance-based method instead of a temperature-based method, and when the surrounding impedance increases during ablation, the power output (wattage) is variably adjusted. This mechanism has already been proven to be safe and effective for thermal treatment of various tumors since the early 2000s [
4]. Because the electrode of VS itself does not generate heat, the catheter can be kept at a low temperature during the ablation process. Furthermore, unlike other existing endovenous RF catheters, the new VS RF catheter has an internal cooling system. Hollow lumens are present inside the catheter, and chilled perfusate circulates in the lumen to continuously cool the catheter. The warmed effluent is removed from the collection unit outside the body. When this internal cooling system is applied, it causes a heat sink effect and removes heat from the area in contact with the electrode [
5]. As the heating of tissues nearest to the electrode decreases, more current can be deposited without tissue charring or impedance increase [
6]. Therefore, it was expected that effective ablation could be achieved during endovenous RFA by preventing the reduction of the therapeutic effect due to carbonization of the electrode and preventing adhesion between the electrode and surrounding venous wall. In addition, the scenario that ablation is possible at low temperatures means that less undesired perivascular thermal injury would occur.
In this study, ablation was performed in 7 dogs for a total of 11 veins using CF and 12 veins using VS. TTC staining was applied to the harvested veins to macroscopically verify whether the veins were actually ablated and whether the viability of the ablated vein has disappeared. TTC staining has been used for the macroscopic evaluation of tissue viability [
78], and the TTC-unstained area is a site without viability. Since unstained areas were identified in all ablated veins using CF and VS, this result confirms that all ablated veins had obvious thermal damage. To objectively compare the endovenous ablation effectiveness of CF and VS, the severity of the damaged layer was graded as a vessel injury score. There has already been a report evaluating the treatment effect on the saphenous vein by comparing the extent of the remaining endothelial cell layer histologically [
9]. The results of the present study revealed no significant difference in vessel injury score between CF and VS (2.54 ± 1.16
vs. 2.42 ± 1.13, P = 0.656). Furthermore, in 1 subject who underwent follow-up, it was confirmed that blood flow persisted in 1 vein where ablation was performed using CF. These results indicate that both catheters produce a similar degree of venous wall damage to show a therapeutic effect and that the venous ablation effect of the new VS catheter is comparable to that of the existing CF catheter.
The vein wall thickness measured by US after ablation was also not significantly different between the 2 devices. It is difficult to mention that the same degree of wall thickening occurs in both types of devices because the pre-procedure vein wall thickness was not measured in advance. However, considering that there is no difference in the result of wall thickness and that the 2 types of devices caused similar vein wall damage histologically, it may be assumed that the effect of ablation results in a similar degree of vein wall thickening.
In an
in vitro model measuring changes in adventitial temperature along the bovine vein during endovenous RF energy delivery, the mean peak adventitial temperature of 64.4 ℃ decreased to 51.3 ℃ when the adventitia was bathed in a 2-mm layer of saline [
10]. Although tumescent anesthesia has a clear advantage of providing thermal protection to perivenous tissue, some degree of bruise or pain caused by venous perforation that occurs during the thermal ablation is inevitable. Theoretically, ablation using a VS RF catheter, which uses a mechanism similar to RFiTT, induces an ablating effect at a lower temperature. Therefore, it can be expected that the RF electrode would result in less undesired perivascular thermal injury. In the present study, a vessel injury score of 4 suggesting disruption of the adventitia, was 21.2% (7 of 33) in the CF group and 11.1% (4 of 36) in the VS group. Based on the experimental result, it is assumed that the VS catheter would cause less perivenous damage than the CF catheter; thus, better safety profile might be expected in future clinical studies.
Since the VS catheter was equipped with an internal cooling system, the catheter temperature itself could be kept lower, assuming that adhesion would not occur. However, in this experimental study, adhesion occurred in 1 subject that underwent ablation using VS catheter without tumescent anesthesia. The event of catheter adherence is considered to be that vessel occlusion was well completed and ablation was terminated early by an impedance-based mechanism; because the carbonized portion was not found when the catheter was removed, and it was confirmed on TTC staining and vessel injury score that the targeted vein was properly ablated.
There are a few limitations in this study. First, although multiple segments of veins could be ablated in 1 dog, the number of dogs was small. Second, the follow-up period was only 14 days. If a longer-term follow-up had been achieved, the reliability of the effectiveness might be increased.
In conclusion, a newly developed VS RF catheter can provide comparable occlusion rate and degree of vein wall injury to that of the most frequently used RF catheter (CF) for endovenous RF ablation in the animal study. Furthermore, it does not induce perivenous adverse event. Endovenous RF ablation using VS catheter with tumescent anesthesia seems to be as effective and safe as CF catheter.
Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download