Abstract
Purpose
Laparoscopic right colectomy (LRC) with extracorporeal anastomosis (ECA) remains the most widely adopted technique despite mounting evidence that intracorporeal anastomosis (ICA) offers several advantages. This study aimed to compare the postoperative outcomes of ICA and ECA and to investigate the effect of ICA on postoperative ileus after LRC.
Methods
This retrospective study included 45 patients who underwent ICA and 63 who underwent ECA in LRC for right-sided colonic diseases between January 2015 and December 2019.
Results
There were no significant differences in total operation time, blood loss, total length of incisions, tolerance of diet, postoperative pain score on postoperative days 1 and 2, or length of hospital stays between the 2 groups. However, the ICA group had a significantly shorter time to first flatus passage (3.0 ± 0.9 days vs. 3.8 ± 1.9 days, P = 0.013). The rate of postoperative ileus was significantly higher in the ECA group (2.2% vs. 14.3%, P = 0.033); however, there was no significant difference in the overall morbidity within 30 days after surgery. Multivariate logistic regression analysis showed that the ECA technique (odds ratio [OR], 0.098; 95% confidence interval [CI]; 0.011–0.883, P = 0.038) and previous abdominal operation (OR, 5.269; 95% CI, 1.193–23.262; P = 0.028) were independent risk factors for postoperative ileus.
Go to : 
Since its introduction in 1991, laparoscopic right colectomy (LRC) has been considered the standard surgical treatment for benign and malignant right colon diseases, and its feasibility in terms of favorable short-term clinical outcomes and oncological radicalism has been widely acknowledged [123]. In addition, a number of studies on complete mesocolic excision with central vascular ligation have demonstrated the feasibility, safety, and acceptability of LRC in terms of postoperative outcomes and oncological profile [4567].
Extracorporeal anastomosis (ECA) remains the most widely adopted laparoscopic anastomotic technique for LRC. Although intracorporeal anastomosis (ICA) in LRC bears certain advantages, including fewer complications related to the alignment of the mesentery and less mobilization of the distal transverse colon for tension-free anastomosis, it has not been widely applied [891011]. In addition, there are concerns that intracorporeal suturing with laparoscopic straight instruments is more difficult and time-consuming than ECA and increases the risk of intraabdominal abscess due to the higher risk of bowel content spillage [12].
Postoperative ileus (POI) is an iatrogenic disorder characterized by the inhibition of gastrointestinal motility following abdominal surgery. Its clinical manifestations include nausea, vomiting, abdominal distention, inability to pass gas, and intolerance to oral intake [913]. Although the definition is not consensual, POI is a leading cause of increased morbidity and prolonged hospital stay [14]. Several studies compared the postoperative outcomes of intracorporeal and ECA in LRC [913]. However, there have been few systematic studies on the effect of the anastomosis technique in LRC on POI, along with the analysis of various factors affecting POI. This study aimed to compare the postoperative outcomes of intracorporeal and extracorporeal anastomoses and to investigate the effect of ICA on POI after LRC.
Go to : 
The study protocol of this study was approved by the Institutional Review Board of the Keimyung University Dongsan Hospital (No. 2022-04-003). The informed consent requirement was waived due to the retrospective manner of the study.
Between January 2015 and December 2019, the study enrolled 119 patients who had undergone LRC for right-sided colonic diseases, including malignancy. Exclusion criteria were as follows: (1) conversion from laparoscopic to open surgery, (2) more than 2 major solid organ surgeries at once, and (3) history of other previous malignancies. After excluding the aforementioned patients, the study included 45 patients who underwent LRC with ICA and 63 patients who underwent ECA.
Patient demographics and perioperative outcomes were obtained from the prospectively collected colorectal cancer database. Patient demographic data included age, sex, preoperative CEA level, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status (PS) classification, and tumor location. Operative outcomes included the total operation time, incision length, blood loss, specimen extraction site, and anastomosis method. Conversion was defined as the transition from laparoscopic to open surgery. Clinical outcomes included postoperative pain management, time to gas pass, sips of water, soft diet, hospital stay, pain score, use of painkillers on postoperative days 1 and 2, postoperative morbidity, and mortality within 30 days. POI was diagnosed when 2 or more of the following 5 criteria were met on or after the 4th postoperative day without resolution of POI: nausea or vomiting, inability to tolerate an oral diet over the previous 24 hours, absence of flatus over the previous 24 hours, abdominal distension, and radiologic confirmation [15]. Morbidity was classified using the Clavien-Dindo (CD) classification. On postoperative days 1 and 2, postoperative wound pain was measured using a numeric pain rating scale, with endpoints labeled “no pain” (scale 0) and “worst possible pain” (scale 10). Pathological outcomes for colonic adenocarcinoma included tumor stage, histology, retrieved lymph nodes, tumor size, and resection margins. Tumors were classified according to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging system.
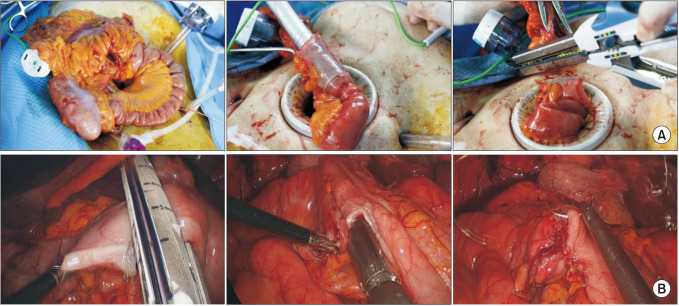
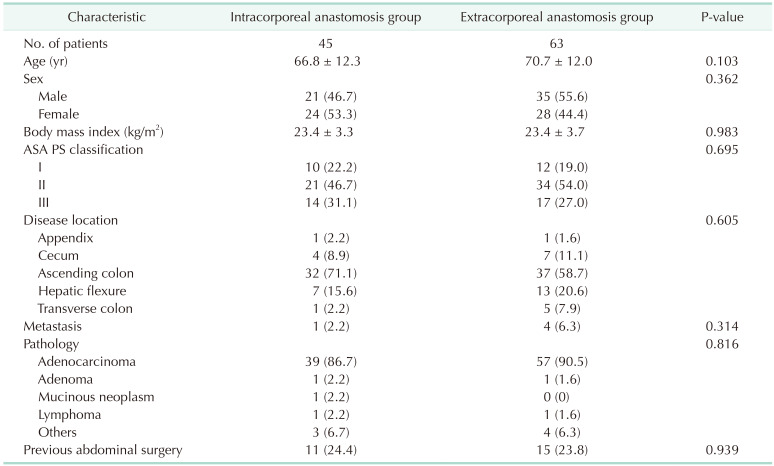
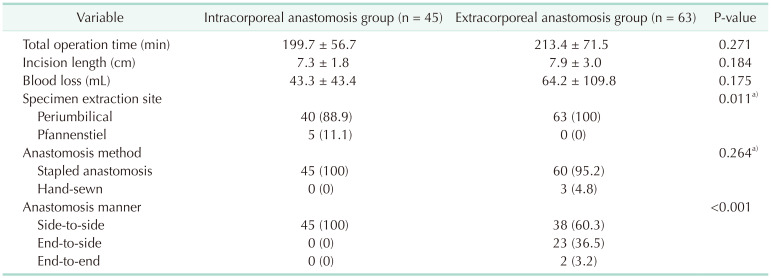
We routinely performed mechanical bowel preparation 1 day before surgery regardless of anastomotic technique. In the ECA group, the mobilized bowel was extracted through a commercial wound protector following a further incision that continued through the previous periumbilical incision (Fig. 1A). An ECA was performed in an end-to-side manner using a circular stapler, side-to-side using a linear stapler or end-to-end with hand-sewn technique. In the ICA group, the transverse mesocolon and small bowel mesentery were divided using a surgical energy device. Subsequently, the transverse colon and terminal ileum were transected using laparoscopic staplers (Fig. 1B). We placed gauze under the anastomotic site to minimize the spread of bowel content into the abdominal cavity during the ICA. Enterotomy and colostomy were performed, and a linear stapler was used to create an isoperistaltic, side-to-side anastomosis. After stapling for anastomosis, sufficient irrigation and suction were performed. The stapler insertion site was closed with continuous stitches using V-Loc sutures (Covidien). The specimen was extracted through a periumbilical or Pfannenstiel incision.
The results are presented as mean ± standard deviation for continuous variables, and as frequencies and percentages for categorical ones. Categorical variables were analyzed using the chi-square and Fisher exact tests. Continuous variables were analyzed using an independent t-test. A P-value of <0.05 was considered to indicate statistical significance. After a univariate analysis to evaluate the risk factors for POI, a multivariate logistic regression analysis was performed with selected variables (with P < 0.10 as inclusion criterion). Statistical analyses were performed using the IBM SPSS Statistics ver. 25 (IBM Corp.).
Go to : 
Preoperative general characteristics of patients, such as age, sex, BMI, ASA PS classification, disease location, and pathologic type of resected specimen, did not show significant differences between the 2 groups (Table 1). In both ICA and ECA groups, the indications for right colectomy were mainly adenocarcinoma (86.7% in the ICA and 90.5% in the ECA). In both groups, the incidence of prior abdominal surgery was comparable.
The operative outcomes are summarized in Table 2. The total operative time was 199.7 minutes in the ICA group and 213.4 minutes in the ECA group (P = 0.271). The total incision length and blood loss were comparable between the 2 groups. In the ICA group, specimens were extracted from the periumbilical site (88.9%) and suprapubic Pfannenstiel incision (11.1%), whereas in the ECA group, specimens were extracted exclusively from the periumbilical incision site (P = 0.011). In the ICA group, all anastomoses were performed using an endolinear stapler, whereas in the ECA group, 60 anastomoses (95.2%) were performed using linear or circular staplers and 3 (4.8%) were performed using the hand-sewn technique (P = 0.264). All 45 anastomoses in the ICA group were performed side-to-side, whereas 60.3%, 36.5%, and 3.2% of extracorporeal anastomoses were performed side-to-side, end-to-side, and end-to-end, respectively (P < 0.001).
In terms of postoperative pain management, there was no significant difference between the 2 groups (P = 0.113) (Table 3). The pain score measured using the numeric rating scale and the number of painkillers on postoperative days 1 and 2 did not show significant differences between the 2 groups. The time to first flatus was significantly shorter in the ICA group than in the ECA group (3.0 ± 0.9 days vs. 3.8 ± 1.9 days, P = 0.013); however, times of water drinking, and soft diet intake were not significantly different between the 2 groups.
The overall postoperative complications within 30 days after surgery were comparable (48.9% in ICA and 46.0% in ECA, P = 0.769), and there was no statistically significant difference between the 2 groups in the proportion of complications of CD classification of III or higher. The incidence of POI in the ICA group was significantly lower than the 1 in the ECA group (2.2% vs. 14.3%; P = 0.033). Three patients in the ICA group, whereas none in the ECA group, developed pseudomembranous colitis (P = 0.033). No mortality was observed in either group for 30 days.
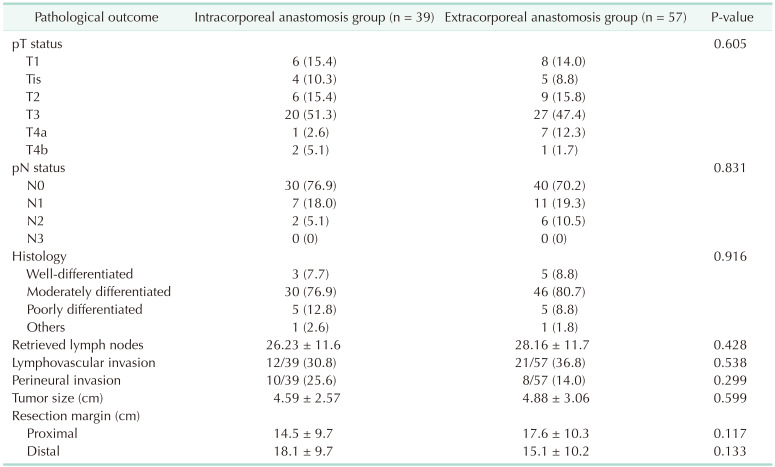
Among the 108 patients, 96 with colonic adenocarcinoma were analyzed for postoperative pathological outcomes. TNM stage (AJCC 8th edition), histologic characteristics, tumor size, lymphovascular invasion, perineural invasion, number of retrieved lymph nodes, and lengths of resection margins were not significantly different between the 2 groups (Table 4).
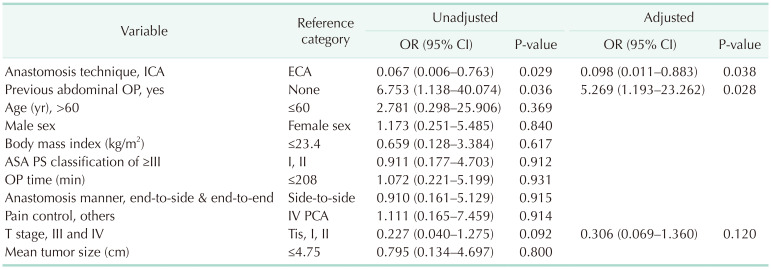
We conducted a univariate analysis with the variables age, sex, BMI, previous abdominal operation history, ASA PS classification, operation time, operation method, anastomosis manner, anastomosis technique, pain control method, T stage, and tumor size, which are thought to influence the POI. During stepwise regression variable selection, the anastomosis technique and history of prior abdominal surgery were identified as potential contributors to POI (P = 0.029 and P = 0.036, respectively). The ECA technique (odds ratio [OR], 0.098; 95% confidence interval [CI], 0.011–0.883; P = 0.038) and prior abdominal surgery (OR, 5.269; 95% CI, 1.193–23.262; P = 0.028) were identified as independent risk factors for POI by multivariate logistic regression analysis (Table 5).
Go to : 
The current study indicated that short-term clinical outcomes, including total operation time, recovery-related outcomes, and overall morbidity, did not differ between the ICA and ECA groups, with the exception of the first flatus passage, which was significantly shorter, and the POI, which was significantly lower in the ICA group than in the ECA group. In the multivariable logistic analysis of risk factors for POI, ECA was assessed as an independent risk factor for POI, in addition to a history of abdominal surgery. These results demonstrate that ICA could take advantages on recovery of the gastrointestinal tract and provides a rationale for using ICA rather than conventional ECA in LRC.
Several comparative studies between ICA and ECA for LRC highlighted the advantages of ICA in terms of shorter hospital stay, quicker bowel recovery, shorter wound length, and fewer postoperative complications [161718]. However, laparoscopic ICA has not been performed extensively because of the inflexibility of intracorporeal suturing devices and requirement of advanced laparoscopic expertise. The safety of ICA has already been acknowledged in the field of gastrectomy [19]. The surgical techniques of ICA during LRC and ICA during laparoscopic gastric resection are similar in that they require suturing common entry during ICA. Regarding the operation time, some studies indicated a longer operative time with technical difficulty in the ICA group, while others reported a shorter total operative time compared to the ECA group [17202122]. Two colorectal surgeons participated in this study, one of whom had undergone colorectal cancer for more than 10 years, and another had been performing colorectal cancer for more than 5 years. Early experiences of ICA performed by 2 surgeons during the study period were included. In our study, there was no significant difference between the 2 groups in terms of the total operation time and anastomotic complications. Considering that the initial experiences of ICA were included in this study, ICA can be said to be safe and feasible.
Anastomotic leakage is one of the most feared complications of colorectal surgery, resulting in longer hospital stay, higher costs, and higher local recurrence and mortality rates. When developing and applying a new anastomosis technique, its complications are typically more important than its benefits. In the current study, there was no difference in anastomotic leakage between the 2 groups. We believe that closing the common entry after side-to-side anastomosis with a linear stapler during ICA does not majorly affect anastomotic leakage, and ICA is safe and feasible compared with conventional ECA.
Leakage of bowel content during ICA is a significant and controversial issue. If bowel preparation is incomplete, the bowel contents may come out of the bowel when an opening for anastomosis is created. This can result in postoperative intraabdominal abscess formation and surgical site infections, both of which are associated with POI. In the present study, there was no significant difference in surgical site infections, including intraabdominal abscesses. Scarborough et al. [23] also reported the preventive role of mechanical bowel preparation and prophylactic antibiotics in postoperative infectious complications. In our practice, we routinely perform mechanical bowel preparation 1 day before surgery, and we place gauze under the anastomotic site to minimize the spread of bowel content into the abdominal cavity during the anastomotic procedure. In addition, sufficient irrigation and suction are performed immediately after anastomosis.
POI can exacerbate patient pain and discomfort, lengthen hospitalization, and place a substantial burden on the healthcare system. The success of enhanced bowel recovery after surgery is directly linked to efforts to reduce POI, including minimally invasive surgeries. Previous studies demonstrated that ICA was associated with superior postoperative recovery outcomes, such as a shorter hospital stay and quicker bowel movements, compared with ECA [17242526]. In the present study, the incidence of POI was significantly lower, and the time to first flatus passage was significantly shorter in the ICA group than in the ECA group [26]. We believe that less traction on the mesentery and less bowel manipulation during ICA could result in lesser POI and quicker bowel recovery. ICA can be aggressively considered in patients with obesity, a thick bowel wall, or a short and thick mesentery requiring an enlarging minilaparotomy.
Some preoperative, intraoperative, and postoperative factors may influence POI. Bragg et al. [13] cited increased age, sex, opioid use, previous abdominal surgery, prolonged operation time, and blood loss as risk factors for POI in their meta-analysis. Kronberg et al. [27] demonstrated that preoperative narcotic use and prior abdominal surgery were independent risk factors for POI after colorectal surgery, and Lee et al. [28] reported a correlation between POI and prior abdominal surgery in colorectal surgery. In our study, the ECA technique and prior major abdominal surgery were independent risk factors for POI, whereas there was no significant difference between the 2 groups with prior abdominal surgery. Although careful interpretation is required, we believe that it is necessary to actively consider the implementation of ICA in patients who have undergone major abdominal surgery in the past, unless minimally invasive surgery is impossible due to severe adhesions.
The current study bears several limitations, including its retrospective nature, small sample size, and selection bias as a result of surgeons choosing the anastomotic technique based on their individual preferences. Each surgical technique is performed in diverse iliocolic anastomosis manner, direct comparison between ICA and ECA in the same manner of anastomosis would need to be more convincing result. Due to the low incidence of events and small sample size, the study may have been underpowered to select appropriate statistical variables for POI. A multicenter study with a larger number of patients would validate the current findings and elucidate the benefits of ICA in greater detail.
In conclusion, the postoperative outcomes of patients who underwent LRC with ICA or ECA were comparable, and ICA could reduce the incidence of POI after LRC compared to ECA.
Go to : 
References
1. Arezzo A, Passera R, Ferri V, Gonella F, Cirocchi R, Morino M. Laparoscopic right colectomy reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Int J Colorectal Dis. 2015; 30:1457–1472. PMID: 26137968.
2. Yang IJ, Seo M, Oh HK, Lee J, Suh JW, Kim DW, et al. Surgical outcomes of single-port laparoscopic surgery compared with conventional laparoscopic surgery for appendiceal mucinous neoplasm. Ann Coloproctol. 2021; 37:239–243. PMID: 34082510.
3. Schlinkert RT. Laparoscopic-assisted right hemicolectomy. Dis Colon Rectum. 1991; 34:1030–1031. PMID: 1834447.
4. Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, et al. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014; 21:2288–2294. PMID: 24604585.
5. Bae SU, Yang SY, Min BS. Totally robotic modified complete mesocolic excision and central vascular ligation for right-sided colon cancer: technical feasibility and mid-term oncologic outcomes. Int J Colorectal Dis. 2019; 34:471–479. PMID: 30560354.
6. Son GM, Lee IY, Lee YS, Kye BH, Cho HM, Jang JH, et al. Is laparoscopic complete mesocolic excision and central vascular ligation really necessary for all patients with right-sided colon cancer? Ann Coloproctol. 2021; 37:434–444. PMID: 34875818.
7. Bae SU, Jeong WK, Baek SK. Single-port laparoscopic complete mesocolic excision and central vascular ligation for caecal cancer with apical lymph node metastasis: a video vignette. Colorectal Dis. 2018; 20:643–644. PMID: 29673043.
8. Frigault J, Avoine S, Drolet S, Letarte F, Bouchard A, Gagné JP, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right hemicolectomy: a retrospective cohort study of anastomotic complications. Ann Coloproctol. 2022; 03. 29. DOI: 10.3393/ac.2021.00983.0140. [Epub].
9. Lee N, Lee SY, Kim CH, Kwak HD, Ju JK, Kim HR. The relationship between high-output stomas, postoperative ileus, and readmission after rectal cancer surgery with diverting ileostomy. Ann Coloproctol. 2021; 37:44–50. PMID: 32972101.
10. Bae SU, Jeong WK, Baek SK. Reduced-port laparoscopic complete mesocolic excision and intracorporeal anastomosis for right-sided colon cancer: a video vignette. Colorectal Dis. 2020; 22:1768–1769. PMID: 32445502.
11. Bae SU, Jeong WK, Baek SK. Robotic complete mesocolic excision and intracorporeal anastomosis using a robotic stapler for right-sided colon cancer with reduced-port access. Dis Colon Rectum. 2017; 60:456. PMID: 28267014.
12. Feroci F, Lenzi E, Garzi A, Vannucchi A, Cantafio S, Scatizzi M. Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2013; 28:1177–1186. PMID: 23371336.
13. Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr. 2015; 34:367–376. PMID: 25819420.
14. Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. 2016; 153:439–446. PMID: 27666979.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383. PMID: 3558716.
16. Allaix ME, Degiuli M, Bonino MA, Arezzo A, Mistrangelo M, Passera R, et al. Intracorporeal or extracorporeal ileocolic anastomosis after laparoscopic right colectomy: a double-blinded randomized controlled trial. Ann Surg. 2019; 270:762–767. PMID: 31592811.
17. Bollo J, Turrado V, Rabal A, Carrillo E, Gich I, Martinez MC, et al. Randomized clinical trial of intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy (IEA trial). Br J Surg. 2020; 107:364–372. PMID: 31846067.
18. Zhang M, Lu Z, Zheng Z, Cheng P, Zhou H, Wang X. Comparison of short-term outcomes between totally laparoscopic right colectomy and laparoscopic-assisted right colectomy: a retrospective study in a single institution on 300 consecutive patients. Surg Endosc. 2022; 36:176–184. PMID: 33427910.
19. Chen K, Mou YP, Xu XW, Pan Y, Zhou YC, Cai JQ, et al. Comparison of short-term surgical outcomes between totally laparoscopic and laparoscopic-assisted distal gastrectomy for gastric cancer: a 10-y single-center experience with meta-analysis. J Surg Res. 2015; 194:367–374. PMID: 25488721.
20. Shapiro R, Keler U, Segev L, Sarna S, Hatib K, Hazzan D. Laparoscopic right hemicolectomy with intracorporeal anastomosis: short- and long-term benefits in comparison with extracorporeal anastomosis. Surg Endosc. 2016; 30:3823–3829. PMID: 26659237.
21. Fabozzi M, Allieta R, Brachet Contul R, Grivon M, Millo P, Lale-Murix E, et al. Comparison of short- and medium-term results between laparoscopically assisted and totally laparoscopic right hemicolectomy: a case-control study. Surg Endosc. 2010; 24:2085–2091. PMID: 20174945.
22. Martinek L, You K, Giuratrabocchetta S, Gachabayov M, Lee K, Bergamaschi R. Does laparoscopic intracorporeal ileocolic anastomosis decreases surgical site infection rate?: a propensity score-matched cohort study. Int J Colorectal Dis. 2018; 33:291–298. PMID: 29327167.
23. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg. 2015; 262:331–337. PMID: 26083870.
24. Vignali A, Bissolati M, De Nardi P, Di Palo S, Staudacher C. Extracorporeal vs. intracorporeal ileocolic stapled anastomoses in laparoscopic right colectomy: an interim analysis of a randomized clinical trial. J Laparoendosc Adv Surg Tech A. 2016; 26:343–348. PMID: 26919037.
25. Carnuccio P, Jimeno J, Parés D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol. 2014; 18:5–12. PMID: 23686680.
26. Creavin B, Balasubramanian I, Common M, McCarrick C, El Masry S, Carton E, et al. Intracorporeal vs extracorporeal anastomosis following neoplastic right hemicolectomy resection: a systematic review and meta-analysis of randomized control trials. Int J Colorectal Dis. 2021; 36:645–656. PMID: 33244717.
27. Kronberg U, Kiran RP, Soliman MS, Hammel JP, Galway U, Coffey JC, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg. 2011; 253:78–81. PMID: 21233608.
28. Lee SY, Kim CH, Kim YJ, Kim HR. Laparoscopic surgery for colorectal cancer patients who underwent previous abdominal surgery. Surg Endosc. 2016; 30:5472–5480. PMID: 27129560.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download